COP1 dynamics integrate conflicting seasonal light and thermal cues in the control of Arabidopsis elongation
- PMID: 35984876
- PMCID: PMC9390991
- DOI: 10.1126/sciadv.abp8412
COP1 dynamics integrate conflicting seasonal light and thermal cues in the control of Arabidopsis elongation
Abstract
As the summer approaches, plants experience enhanced light inputs and warm temperatures, two environmental cues with an opposite morphogenic impact. Key components of this response are PHYTOCHROME B (phyB), EARLY FLOWERING 3 (ELF3), and CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1). Here, we used single and double mutant/overexpression lines to fit a mathematical model incorporating known interactions of these regulators. The fitted model recapitulates thermal growth of all lines used and correctly predicts thermal behavior of others not used in the fit. While thermal COP1 function is accepted to be independent of diurnal timing, our model shows that it acts at temperature signaling only during daytime. Defective response of cop1-4 mutants is epistatic to phyB-9 and elf3-8, indicating that COP1 activity is essential to transduce phyB and ELF3 thermosensory function. Our thermal model provides a unique toolbox to identify best allelic combinations enhancing climate change resilience of crops adapted to different latitudes.
Figures



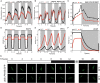

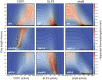
References
-
- Casal J. J., Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427 (2013). - PubMed
-
- Gommers C. M. M., Visser E. J. W., Onge K. R. S., Voesenek L. A. C. J., Pierik R., Shade tolerance: When growing tall is not an option. Trends Plant Sci. 18, 65–71 (2013). - PubMed
-
- Jung J.-H., Domijan M., Klose C., Biswas S., Ezer D., Gao M., Khattak A. K., Box M. S., Charoensawan V., Cortijo S., Kumar M., Grant A., Locke J. C. W., Schäfer E., Jaeger K. E., Wigge P. A., Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889 (2016). - PubMed
-
- Legris M., Klose C., Burgie E. S., Rojas C. C. R., Neme M., Hiltbrunner A., Wigge P. A., Schäfer E., Vierstra R. D., Casal J. J., Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354, 897–900 (2016). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

