Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC
- PMID: 35985289
- PMCID: PMC9630439
- DOI: 10.1016/j.cell.2022.07.010
Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC
Abstract
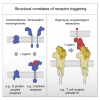
The T cell receptor (TCR) expressed by T lymphocytes initiates protective immune responses to pathogens and tumors. To explore the structural basis of how TCR signaling is initiated when the receptor binds to peptide-loaded major histocompatibility complex (pMHC) molecules, we used cryogenic electron microscopy to determine the structure of a tumor-reactive TCRαβ/CD3δγε2ζ2 complex bound to a melanoma-specific human class I pMHC at 3.08 Å resolution. The antigen-bound complex comprises 11 subunits stabilized by multivalent interactions across three structural layers, with clustered membrane-proximal cystines stabilizing the CD3-εδ and CD3-εγ heterodimers. Extra density sandwiched between transmembrane helices reveals the involvement of sterol lipids in TCR assembly. The geometry of the pMHC/TCR complex suggests that efficient TCR scanning of pMHC requires accurate pre-positioning of T cell and antigen-presenting cell membranes. Comparisons of the ligand-bound and unliganded receptors, along with molecular dynamics simulations, indicate that TCRs can be triggered in the absence of spontaneous structural rearrangements.
Keywords: adaptive immunity; antigen presentation; electron microscopy; membrane proteins; receptor triggering; structural cell biology; supramolecular complexes.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




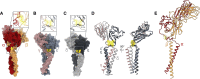
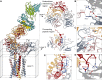






References
-
- Abraham M.J., Murtola R., Schulz R., Páil S., Smith J.C., Hess B., Lindathl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputer. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Beddoe T., Chen Z., Clements C.S., Ely L.K., Bushell S.R., Vivian J.P., Kjer-Nielsen L., Pang S.S., Dunstone M.A., Liu Y.C., et al. Antigen ligation triggers a conformational change within the constant domain of the alphabeta T cell receptor. Immunity. 2009;30:777–788. doi: 10.1016/j.immuni.2009.03.018. - DOI - PubMed
-
- Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., DiNola A., Haak J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

