Neuroblastoma: When differentiation goes awry
- PMID: 35985323
- PMCID: PMC9509448
- DOI: 10.1016/j.neuron.2022.07.012
Neuroblastoma: When differentiation goes awry
Abstract
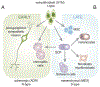
Neuroblastoma is a leading cause of cancer-related death in children. Accumulated data suggest that differentiation arrest of the neural-crest-derived sympathoadrenal lineage contributes to neuroblastoma formation. The developmental arrest of these cell types explains many biological features of the disease, including its cellular heterogeneity, mutational spectrum, spontaneous regression, and response to drugs that induce tumor cell differentiation. In this review, we provide evidence that supports the notion that arrested neural-crest-derived progenitor cells give rise to neuroblastoma and discuss how this concept could be exploited for clinical management of the disease.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
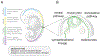


References
-
- Anderson DJ (1993). Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci 16, 129–158. - PubMed
-
- Bailey P (1926). A classification of the tumors of the glioma group on a histogenetic basis with a correlated study of prognosis (Philadelphia: J.B. Lippincott Company; ).
-
- Bechmann N, Berger I, Bornstein SR, and Steenblock C (2021). Adrenal medulla development and medullary-cortical interactions. Mol Cell Endocrinol 528, 111258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

