Rapid tissue prototyping with micro-organospheres
- PMID: 35985334
- PMCID: PMC9481922
- DOI: 10.1016/j.stemcr.2022.07.016
Rapid tissue prototyping with micro-organospheres
Abstract
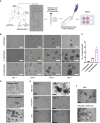
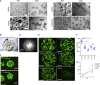
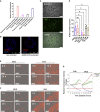
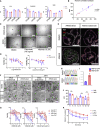
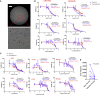
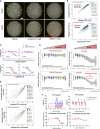
In vitro tissue models hold great promise for modeling diseases and drug responses. Here, we used emulsion microfluidics to form micro-organospheres (MOSs), which are droplet-encapsulated miniature three-dimensional (3D) tissue models that can be established rapidly from patient tissues or cells. MOSs retain key biological features and responses to chemo-, targeted, and radiation therapies compared with organoids. The small size and large surface-to-volume ratio of MOSs enable various applications including quantitative assessment of nutrient dependence, pathogen-host interaction for anti-viral drug screening, and a rapid potency assay for chimeric antigen receptor (CAR)-T therapy. An automated MOS imaging pipeline combined with machine learning overcomes plating variation, distinguishes tumorspheres from stroma, differentiates cytostatic versus cytotoxic drug effects, and captures resistant clones and heterogeneity in drug response. This pipeline is capable of robust assessments of drug response at individual-tumorsphere resolution and provides a rapid and high-throughput therapeutic profiling platform for precision medicine.
Keywords: CAR-T; SARS-COV-2; cytostatic; cytotoxic; deep learning; demulsification; drug resistant; micro-organospheres; organoid; patient derived organoid.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest X.S., D.H., and H.C. are co-founders of Xilis, Inc. X.S. left Duke and joined Terasaki Institute and Xilis on November 9, 2021. H.C. is also a member of the board of directors of Roche. H.C.’s full disclosure is given at https://www.uu.nl/staff/JCClevers/. Z.W. recently left Duke University and joined Xilis, Inc. as a full-time employee. Patents WO2020242594, US 2021/0285054, and US 2022/006279 are related to this work.
Figures

References
-
- Artegiani B., Hendriks D., Beumer J., Kok R., Zheng X., Joore I., Chuva de Sousa Lopes S., van Zon J., Tans S., Clevers H. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. Nat. Cell Biol. 2020;22:321–331. doi: 10.1038/s41556-020-0472-5. - DOI - PubMed
-
- Beumer J., Artegiani B., Post Y., Reimann F., Gribble F., Nguyen T.N., Zeng H., Van den Born M., Van Es J.H., Clevers H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018;20:909–916. doi: 10.1038/s41556-018-0143-y. - DOI - PMC - PubMed
-
- Boretto M., Maenhoudt N., Luo X., Hennes A., Boeckx B., Bui B., Heremans R., Perneel L., Kobayashi H., Van Zundert I., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019;21:1041–1051. doi: 10.1038/s41556-019-0360-z. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

