Oxypeucedanin relieves LPS-induced acute lung injury by inhibiting the inflammation and maintaining the integrity of the lung air-blood barrier
- PMID: 35985771
- PMCID: PMC9467393
- DOI: 10.18632/aging.204235
Oxypeucedanin relieves LPS-induced acute lung injury by inhibiting the inflammation and maintaining the integrity of the lung air-blood barrier
Abstract
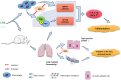
Introduction: Acute lung injury (ALI) is commonly accompanied by a severe inflammatory reaction process, and effectively managing inflammatory reactions is an important therapeutic approach for alleviating ALI. Macrophages play an important role in the inflammatory response, and this role is proinflammatory in the early stages of inflammation and anti-inflammatory in the late stages. Oxypeucedanin is a natural product with a wide range of pharmacological functions. This study aimed to determine the effect of oxypeucedanin on lipopolysaccharide (LPS)-induced ALI.
Methods and results: In this study, the following experiments were performed based on LPS-induced models in vivo and in vitro. Using myeloperoxidase activity measurement, ELISA, qRT-PCR, and Western blotting, we found that oxypeucedanin modulated the activity of myeloperoxidase and decreased the expression levels of inflammatory mediators such as TNF-α, IL-6, IL-1β, MPO, COX-2 and iNOS in LPS-induced inflammation models. Meanwhile, oxypeucedanin inhibited the activation of PI3K/AKT and its downstream NF-κB and MAPK signaling pathways. In addition, oxypeucedanin significantly decreased the pulmonary vascular permeability, which was induced by LPSs, and the enhanced expression of tight junction proteins (Occludin and Claudin 3).
Conclusions: In conclusion, this study demonstrated that the anti-inflammatory mechanism of oxypeucedanin is associated with the inhibition of the activation of PI3K/AKT/NF-κB and MAPK signaling pathways and the maintenance of the integrity of the lung air-blood barrier.
Keywords: MAPKs; PI3K/AKT/NF-κB; acute lung injury; lipopolysaccharide; molecular docking simulation.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

