Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance
- PMID: 35985814
- PMCID: PMC9731629
- DOI: 10.1681/ASN.2022030378
Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance
Abstract
Background: CKD is characterized by a sustained proinflammatory response of the immune system, promoting hypertension and cardiovascular disease. The underlying mechanisms are incompletely understood but may be linked to gut dysbiosis. Dysbiosis has been described in adults with CKD; however, comorbidities limit CKD-specific conclusions.
Methods: We analyzed the fecal microbiome, metabolites, and immune phenotypes in 48 children (with normal kidney function, CKD stage G3-G4, G5 treated by hemodialysis [HD], or kidney transplantation) with a mean±SD age of 10.6±3.8 years.
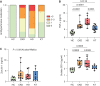
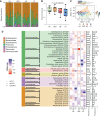
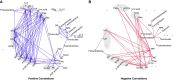
Results: Serum TNF-α and sCD14 were stage-dependently elevated, indicating inflammation, gut barrier dysfunction, and endotoxemia. We observed compositional and functional alterations of the microbiome, including diminished production of short-chain fatty acids. Plasma metabolite analysis revealed a stage-dependent increase of tryptophan metabolites of bacterial origin. Serum from patients on HD activated the aryl hydrocarbon receptor and stimulated TNF-α production in monocytes, corresponding to a proinflammatory shift from classic to nonclassic and intermediate monocytes. Unsupervised analysis of T cells revealed a loss of mucosa-associated invariant T (MAIT) cells and regulatory T cell subtypes in patients on HD.
Conclusions: Gut barrier dysfunction and microbial metabolite imbalance apparently mediate the proinflammatory immune phenotype, thereby driving the susceptibility to cardiovascular disease. The data highlight the importance of the microbiota-immune axis in CKD, irrespective of confounding comorbidities.
Keywords: cardiovascular disease; children; chronic inflammation; chronic kidney disease; dysbiosis; hypertension; immunology; pediatric nephrology; vascular disease.
Copyright © 2022 by the American Society of Nephrology.
Figures





References
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. : Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 - PubMed
-
- Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M: Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res 42: 123–140, 2019 - PubMed
-
- Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. : Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

