STAT1 Contributes to Microglial/Macrophage Inflammation and Neurological Dysfunction in a Mouse Model of Traumatic Brain Injury
- PMID: 35985835
- PMCID: PMC9525171
- DOI: 10.1523/JNEUROSCI.0682-22.2022
STAT1 Contributes to Microglial/Macrophage Inflammation and Neurological Dysfunction in a Mouse Model of Traumatic Brain Injury
Abstract
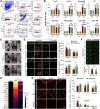
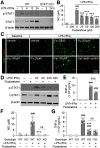
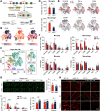
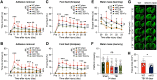
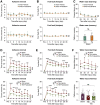
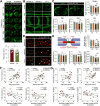
Traumatic brain injury (TBI) triggers a plethora of inflammatory events in the brain that aggravate secondary injury and impede tissue repair. Resident microglia (Mi) and blood-borne infiltrating macrophages (MΦ) are major players of inflammatory responses in the post-TBI brain and possess high functional heterogeneity. However, the plasticity of these cells has yet to be exploited to develop therapies that can mitigate brain inflammation and improve the outcome after TBI. This study investigated the transcription factor STAT1 as a key determinant of proinflammatory Mi/MΦ responses and aimed to develop STAT1 as a novel therapeutic target for TBI using a controlled cortical impact model of TBI on adult male mice. TBI induced robust upregulation of STAT1 in the brain at the subacute injury stage, which occurred primarily in Mi/MΦ. Intraperitoneal administration of fludarabine, a selective STAT1 inhibitor, markedly alleviated proinflammatory Mi/MΦ responses and brain inflammation burden after TBI. Such phenotype-modulating effects of fludarabine on post-TBI Mi/MΦ were reproduced by tamoxifen-induced, selective KO of STAT1 in Mi/MΦ (STAT1 mKO). By propelling Mi/MΦ away from a detrimental proinflammatory phenotype, STAT1 mKO was sufficient to reduce long-term neurologic deficits and brain lesion size after TBI. Importantly, short-term fludarabine treatment after TBI elicited long-lasting improvement of TBI outcomes, but this effect was lost on STAT1 mKO mice. Together, our study provided the first line of evidence that STAT1 causatively determines the proinflammatory phenotype of brain Mi/MΦ after TBI. We also showed promising preclinical data supporting the use of fludarabine as a novel immunomodulating therapy to TBI.SIGNIFICANCE STATEMENT The functional phenotype of microglia and macrophages (Mi/MΦ) critically influences brain inflammation and the outcome after traumatic brain injury (TBI); however, no therapies have been developed to modulate Mi/MΦ functions to treat TBI. Here we report, for the first time, that the transcription factor STAT1 is a key mediator of proinflammatory Mi/MΦ responses in the post-TBI brain, the specific deletion of which ameliorates neuroinflammation and improves long-term functional recovery after TBI. We also show excellent efficacy of a selective STAT1 inhibitor fludarabine against TBI-induced functional deficits and brain injury using a mouse model, presenting STAT1 as a promising therapeutic target for TBI.
Keywords: behavioral test; conditional gene KO; controlled cortical impact; fludarabine; neuroinflammation; signal transducer and activator of transcription 1.
Copyright © 2022 the authors.
Figures

References
-
- Barrett JP, Henry RJ, Shirey KA, Doran SJ, Makarevich OD, Ritzel RM, Meadows VA, Vogel SN, Faden AI, Stoica BA, Loane DJ (2020) Interferon-beta plays a detrimental role in experimental traumatic brain injury by enhancing neuroinflammation that drives chronic neurodegeneration. J Neurosci 40:2357–2370. 10.1523/JNEUROSCI.2516-19.2020 - DOI - PMC - PubMed
-
- Berlex Canada (2003) FLUDARA® product monograph. Pointe-Claire, Quebec.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
