Flavin-enabled reductive and oxidative epoxide ring opening reactions
- PMID: 35986005
- PMCID: PMC9391479
- DOI: 10.1038/s41467-022-32641-1
Flavin-enabled reductive and oxidative epoxide ring opening reactions
Abstract
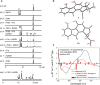
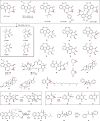
Epoxide ring opening reactions are common and important in both biological processes and synthetic applications and can be catalyzed in a non-redox manner by epoxide hydrolases or reductively by oxidoreductases. Here we report that fluostatins (FSTs), a family of atypical angucyclines with a benzofluorene core, can undergo nonenzyme-catalyzed epoxide ring opening reactions in the presence of flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NADH). The 2,3-epoxide ring in FST C is shown to open reductively via a putative enol intermediate, or oxidatively via a peroxylated intermediate with molecular oxygen as the oxidant. These reactions lead to multiple products with different redox states that possess a single hydroxyl group at C-2, a 2,3-vicinal diol, a contracted five-membered A-ring, or an expanded seven-membered A-ring. Similar reactions also take place in both natural products and other organic compounds harboring an epoxide adjacent to a carbonyl group that is conjugated to an aromatic moiety. Our findings extend the repertoire of known flavin chemistry that may provide new and useful tools for organic synthesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The family of berberine bridge enzyme-like enzymes: A treasure-trove of oxidative reactions.Arch Biochem Biophys. 2017 Oct 15;632:88-103. doi: 10.1016/j.abb.2017.06.023. Epub 2017 Jul 1. Arch Biochem Biophys. 2017. PMID: 28676375 Review.
-
Mutagenesis of the redox-active disulfide in mercuric ion reductase: catalysis by mutant enzymes restricted to flavin redox chemistry.Biochemistry. 1989 Feb 7;28(3):1168-83. doi: 10.1021/bi00429a035. Biochemistry. 1989. PMID: 2653436
-
Coenzyme A-dependent aerobic metabolism of benzoate via epoxide formation.J Biol Chem. 2010 Jul 2;285(27):20615-24. doi: 10.1074/jbc.M110.124156. Epub 2010 May 7. J Biol Chem. 2010. PMID: 20452977 Free PMC article.
-
Catalysis of a flavoenzyme-mediated amide hydrolysis.J Am Chem Soc. 2010 Apr 28;132(16):5550-1. doi: 10.1021/ja9107676. J Am Chem Soc. 2010. PMID: 20369853 Free PMC article.
-
Exploring the synthetic potential of epoxide ring opening reactions toward the synthesis of alkaloids and terpenoids: a review.RSC Adv. 2024 Apr 23;14(19):13100-13128. doi: 10.1039/d4ra01834f. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38655462 Free PMC article. Review.
Cited by
-
Study about fluostatins: efficient preparation of fluostatin A and discovery of fluostatin derivatives from Streptomyces sp. TA-3391.J Antibiot (Tokyo). 2025 Aug;78(9):552-559. doi: 10.1038/s41429-025-00841-8. Epub 2025 Jun 18. J Antibiot (Tokyo). 2025. PMID: 40533539
-
Advances in the synthesis and applications of macrocyclic polyamines.R Soc Open Sci. 2024 Jun 19;11(6):231979. doi: 10.1098/rsos.231979. eCollection 2024 Jun. R Soc Open Sci. 2024. PMID: 39092147 Free PMC article. Review.
-
Biochemical and structural insights of multifunctional flavin-dependent monooxygenase FlsO1-catalyzed unexpected xanthone formation.Nat Commun. 2022 Sep 14;13(1):5386. doi: 10.1038/s41467-022-33131-0. Nat Commun. 2022. PMID: 36104338 Free PMC article.
-
Isolation and NMR Scaling Factors for the Structure Determination of Lobatolide H, a Flexible Sesquiterpene from Neurolaena lobata.Int J Mol Sci. 2023 Mar 19;24(6):5841. doi: 10.3390/ijms24065841. Int J Mol Sci. 2023. PMID: 36982924 Free PMC article.
References
-
- Meninno S, Lattanzi A. Organocatalytic asymmetric reactions of epoxides: recent progress. Chem. Eur. J. 2016;22:3632–3642. - PubMed
-
- He J, Ling J, Chiu P. Vinyl epoxides in organic synthesis. Chem. Rev. 2014;114:8037–8128. - PubMed
-
- Parker RE, Isaacs NS. Mechanisms of epoxide reactions. Chem. Rev. 1959;59:737–799.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

