A Population Pharmacokinetic Modelling Approach to Unravel the Complex Pharmacokinetics of Vincristine in Children
- PMID: 35986122
- PMCID: PMC9556337
- DOI: 10.1007/s11095-022-03364-1
A Population Pharmacokinetic Modelling Approach to Unravel the Complex Pharmacokinetics of Vincristine in Children
Abstract
Background: Vincristine, a chemotherapeutic agent that extensively binds to β-tubulin, is commonly dosed at 1.4-2.0 mg/m2 capped at 2 mg. For infants, doses vary from 0.025-0.05 mg/kg or 50-80% of the mg/m2 dose. However, evidence for lower doses in infants compared to older children is lacking. This study was conducted to unravel the complex pharmacokinetics of vincristine, including the effects of age, to assist optimal dosing in this population.
Methods: 206 patients (0.04-33.9 years; 25 patients < 1 years), receiving vincristine, with 1297 plasma concentrations were included. Semi-mechanistic population pharmacokinetic analyses were performed using non-linear mixed effects modelling.
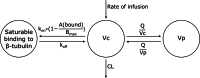
Results: A three-compartment model, with one saturable compartment resembling saturable binding to β-tubulin and thus, saturable distribution, best described vincristine pharmacokinetics. Body weight and age were covariates significantly influencing the maximal binding capacity to β-tubulin, which increased with increasing body weight and decreased with increasing age. Vincristine clearance (CL) was estimated as 30.6 L/h (95% confidence interval (CI) 27.6-33.0), intercompartmental CL (Q) as 63.2 L/h (95%CI 57.2-70.1), volume of distribution of the central compartment as 5.39 L (95%CI 4.23-6.46) and of the peripheral compartment as 400 L (95%CI 357-463) (all parameters correspond to a patient of 70 kg). The maximal binding capacity was 0.525 mg (95%CI 0.479-0.602) (for an 18 year old patient of 70 kg), with a high association rate constant, fixed at 1300 /h and a dissociation constant of 11.5 /h.
Interpretation: A decrease of vincristine β-tubulin binding capacity with increasing age suggests that young children tolerate higher doses of vincristine.
Keywords: oncology; pediatric; pharmacokinetics; population pharmacokinetics.
© 2022. The Author(s).
Conflict of interest statement
There are no conflicts of interests to declare.
Figures

References
-
- van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit Rev Oncol Hematol [Internet]. 2017;114:114–30. Available from: 10.1016/j.critrevonc.2017.04.004 - PubMed
-
- Triarico S, Romano A, Attinà G, Capozza MA, Maurizi P, Mastrangelo S, et al. Vincristine-Induced Peripheral Neuropathy (VIPN) in Pediatric Tumors: Mechanisms, Risk Factors, Strategies of Prevention and Treatment. Int J Mol Sci [Internet]. 2021;22(8):4112. Available from: https://www.mdpi.com/1422-0067/22/8/4112 - PMC - PubMed
-
- Legha SS. Vincristine Neurotoxicity. Med Toxicol [Internet]. 1986;1(6):421–7. Available from: http://link.springer.com/10.1007/BF03259853 - DOI - PubMed
-
- Teva. Summary of product characteristics: Vincristine sulphate (NL). 2020; Available from: https://www.geneesmiddeleninformatiebank.nl/smpc/h100081_smpc.pdf
-
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet [Internet]. 2007;370(9583):240–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S014067360761126X - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

