Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties
- PMID: 35986305
- PMCID: PMC9389821
- DOI: 10.1186/s13287-022-03128-z
Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties
Abstract
Purpose: Extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs) have been demonstrated to possess great potential in preclinical models. An efficient biomanufacturing platform is necessary for scale up production for clinical therapeutic applications. The aim of this study is to investigate the potential differences in neuro-regenerative properties of MSC-derived EVs generated in 2D versus 3D culture systems.
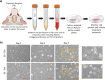
Method: Human bone marrow MSCs (BM-MSCs) were cultured in 2D monolayer and 3D bioreactor systems. EVs were isolated using ultracentrifugation followed by size and concentration measurements utilizing dynamic light scattering (NanoSight) and by fluorescence staining (ExoView). Mouse trigeminal ganglia (TG) neurons were isolated from BALB/c mice and cultured in the presence or absence of EVs derived from 2D or 3D culture systems. Neuronal growth and morphology were monitored over 5 days followed by immunostaining for β3 tubulin. Confocal images were analyzed by Neurolucida software to obtain the density and length of the neurites.
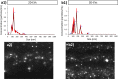
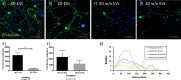
Results: The NanoSight tracking analysis revealed a remarkable increase (24-fold change) in the concentration of EVs obtained from the 3D versus 2D culture condition. ExoView analysis showed a significantly higher concentration of CD63, CD81, and CD9 markers in the EVs derived from 3D versus 2D conditions. Furthermore, a notable shift toward a more heterogeneous phenotype was observed in the 3D-derived EVs compared to those from 2D culture systems. EVs derived from both culture conditions remarkably induced neurite growth and elongation after 5 days in culture compared to untreated control. Neurolucida analysis of the immunostaining images (β3 tubulin) showed a significant increase in neurite length in TG neurons treated with 3D- versus 2D-derived EVs (3301.5 μm vs. 1860.5 μm, P < 0.05). Finally, Sholl analysis demonstrated a significant increase in complexity of the neuronal growth in neurons treated with 3D- versus 2D-derived EVs (P < 0.05).
Conclusion: This study highlights considerable differences in EVs obtained from different culture microenvironments, which could have implications for their therapeutic effects and potency. The 3D culture system seems to provide a preferred environment that modulates the paracrine function of the cells and the release of a higher number of EVs with enhanced biophysical properties and functions in the context of neurite elongation and growth.
Keywords: 2D culture; 3D culture; Bone marrow mesenchymal stromal cell; Exosomes; Extracellular vesicles; Neuronal growth.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

