On the difficulties of characterizing weak protein interactions that are critical for neurotransmitter release
- PMID: 35986639
- PMCID: PMC9623538
- DOI: 10.1002/2211-5463.13473
On the difficulties of characterizing weak protein interactions that are critical for neurotransmitter release
Abstract
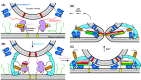
The mechanism of neurotransmitter release has been extensively characterized, showing that vesicle fusion is mediated by the SNARE complex formed by syntaxin-1, SNAP-25 and synaptobrevin. This complex is disassembled by N-ethylmaleimide sensitive factor (NSF) and SNAPs to recycle the SNAREs, whereas Munc18-1 and Munc13s organize SNARE complex assembly in an NSF-SNAP-resistant manner. Synaptotagmin-1 acts as the Ca2+ sensor that triggers exocytosis in a tight interplay with the SNAREs and complexins. Here, we review technical aspects associated with investigation of protein interactions underlying these steps, which is hindered because the release machinery is assembled between two membranes and is highly dynamic. Moreover, weak interactions, which are difficult to characterize, play key roles in neurotransmitter release, for instance by lowering energy barriers that need to be overcome in this highly regulated process. We illustrate the crucial role that structural biology has played in uncovering mechanisms underlying neurotransmitter release, but also discuss the importance of considering the limitations of the techniques used, including lessons learned from research in our lab and others. In particular, we emphasize: (a) the promiscuity of some protein sequences, including membrane-binding regions that can mediate irrelevant interactions with proteins in the absence of their native targets; (b) the need to ensure that weak interactions observed in crystal structures are biologically relevant; and (c) the limitations of isothermal titration calorimetry to analyze weak interactions. Finally, we stress that even studies that required re-interpretation often helped to move the field forward by improving our understanding of the system and providing testable hypotheses.
Keywords: SNAREs; complexin; membrane fusion; neurotransmitter release; synaptotagmin; weak protein interactions.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

