Prophylactic platelet transfusions versus no prophylaxis in hospitalized patients with thrombocytopenia: A systematic review with meta-analysis
- PMID: 35986657
- PMCID: PMC9805167
- DOI: 10.1111/trf.17064
Prophylactic platelet transfusions versus no prophylaxis in hospitalized patients with thrombocytopenia: A systematic review with meta-analysis
Keywords: adverse effects; bleeding; mortality; platelet transfusion; thrombocytopenia.
Conflict of interest statement
The Department of Intensive Care at Rigshospitalet (CA, AG, PS, MHM, AP, and LR) has received funding for other projects from the Novo Nordisk Foundation, Pfizer, Sygeforsikringen “danmark” and Fresenius Kabi and conducts contract research for AM‐Pharma. FP has received personal fees from Gilead and an institutional grant from Alexion for other projects. KP and NZ have no conflict of interests.
Figures
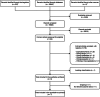
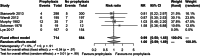
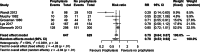

References
-
- Ten Berg MJ, Van Den Bemt PMLA, Shantakumar S, Bennett D, Voest EE, Huisman A, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital‐based cohort study. Drug Saf. 2011;34(12):1151–60. - PubMed
-
- Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–7. - PubMed
-
- Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

