Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: a phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study
- PMID: 35986699
- PMCID: PMC10092158
- DOI: 10.1111/bjd.21826
Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: a phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study
Abstract
Background: Atopic dermatitis (AD) is a prevalent inflammatory, pruritic skin disease. The Janus kinase (JAK) pathway is a treatment target.
Objectives: To assess the efficacy, safety and pharmacokinetics of topical cream brepocitinib, a small-molecule tyrosine kinase 2 (TYK2)/JAK1 inhibitor, in participants with mild-to-moderate AD.
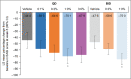
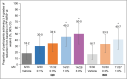
Methods: In this phase IIb, double-blind, dose-ranging study, participants were randomized to receive one of eight treatments for 6 weeks: brepocitinib 0·1% once daily (QD), 0·3% QD or twice daily (BID), 1·0% QD or BID, 3·0% QD, or vehicle QD or BID. The primary endpoint was the percentage change from baseline in the Eczema Area and Severity Index (EASI) total score at week 6. Adverse events (AEs) were monitored.
Results: Overall, 292 participants were enrolled and randomized. The brepocitinib 1% QD and 1% BID groups achieved statistically significantly greater (with multiplicity-adjusted P < 0·05 due to Hochberg's step-up method) percentage reductions from baseline in EASI total score at week 6 [least squares mean (90% confidence interval, CI): QD: -70·1 (-82·1 to -58·0); BID: -75·0 (-83·8 to -66·2)] compared with respective vehicle [QD: -44·4 (-57·3 to -31·6); BID: -47·6 (-57·5 to -37·7)]. There was not a dose-dependent trend in AE frequency, and there were no serious AEs or deaths.
Conclusions: Topical brepocitinib is effective and well tolerated in participants with mild-to-moderate AD. What is already known about this topic? Janus kinase (JAK) inhibitors are in development for treatment of atopic dermatitis (AD). The tyrosine kinase 2 and JAK 1 inhibition by brepocitinib may bring a new profile for topical JAK inhibitors for treatment of mild-to-moderate AD. What does this study add? Topical brepocitinib can provide rapid, effective symptom reduction, and could offer a novel alternative to current topical treatments for mild-to-moderate AD.
© 2022 Pfizer Inc and The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
M.N.L. has served as Principal Investigator and/or Sub‐Investigator for Amgen, Celgene, Cutanea, Eli Lilly, Foamix, Galderma, Incyte, Janssen, Johnson & Johnson, Kadmon, Leo Pharma, Novartis, Novum, Pfizer Inc and Symbio; and has received fees from Amgen, Celgene, Cutanea, Foamix, Galderma, Incyte, Johnson & Johnson, Kadmon, Leo Pharma, Novartis, Novum and Symbio. S.S. has performed clinical research studies for AbbVie, Aclaris Therapeutics, Allergan, Arcutis, Boehringer Ingelheim, Brickell Biotech, Croma Pharma, Dermira, Eli Lilly, Endo Pharmaceuticals, Evolus, Galderma, Glenmark Pharmaceuticals, Leo Pharma, Nielsen Biosciences, Novartis, Pfizer Inc, Prollenium Medical Technologies, Revance Therapeutics and Sun Pharmaceutical Industries; and has received fees and honoraria as a consultant to Brickell Biotech, Galderma, Nielsen Biosciences, Prollenium Medical Technologies, Scarless Laboratories and Teoxane. Z.D. received a research grant from Pfizer Inc to conduct the research detailed in this manuscript. S.T., V.P., S.A., C.B., E.P., M.S.V. and J.X. are employees and stockholders of Pfizer Inc. V.S. and J.S.B. were employees of Pfizer Inc at the time this work was conducted and hold shares in Pfizer Inc. M.A. and L.U. have no conflicts of interest to declare.
Figures

Comment in
-
How will brepocitinib cream compare with established treatments for mild-to-moderate eczema?Br J Dermatol. 2022 Dec;187(6):838. doi: 10.1111/bjd.21880. Epub 2022 Oct 5. Br J Dermatol. 2022. PMID: 36199210 No abstract available.
References
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. - PubMed
-
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. J Allergy Clin Immunol 2013; 132:1132–8. - PubMed
-
- Hebert AA, Stingl G, Ho LK et al. Patient impact and economic burden of mild‐to‐moderate atopic dermatitis. Curr Med Res Opin 2018; 34:2177–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

