Enhanced excitability of the hippocampal CA2 region and its contribution to seizure activity in a mouse model of temporal lobe epilepsy
- PMID: 35987207
- PMCID: PMC9547935
- DOI: 10.1016/j.neuron.2022.07.020
Enhanced excitability of the hippocampal CA2 region and its contribution to seizure activity in a mouse model of temporal lobe epilepsy
Abstract
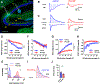
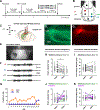
The hippocampal CA2 region, an area important for social memory, has been suspected to play a role in temporal lobe epilepsy (TLE) because of its resistance to degeneration observed in neighboring CA1 and CA3 regions in both humans and rodent models of TLE. However, little is known about whether alterations in CA2 properties promote seizure generation or propagation. Here, we addressed the role of CA2 using the pilocarpine-induced status epilepticus model of TLE. Ex vivo electrophysiological recordings from acute hippocampal slices revealed a set of coordinated changes that enhance CA2 PC intrinsic excitability, reduce CA2 inhibitory input, and increase CA2 excitatory output to its major CA1 synaptic target. Moreover, selective chemogenetic silencing of CA2 pyramidal cells caused a significant decrease in the frequency of spontaneous seizures measured in vivo. These findings provide the first evidence that CA2 actively contributes to TLE seizure activity and may thus be a promising therapeutic target.
Keywords: CA2; designer receptors activated by designer drugs; electroencephalogram; epilepsy; hippocampus; hyperexcitability; inhibitory-excitatory balance; optogenetics; slice electrophysiology; status epilepticus.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




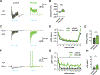

Comment in
-
Et tu, CA2: CA2 Is Hyperexcitable and Controls Seizures in a Mouse Model of Temporal Lobe Epilepsy.Epilepsy Curr. 2023 Jan 18;23(2):121-123. doi: 10.1177/15357597221150068. eCollection 2023 Mar-Apr. Epilepsy Curr. 2023. PMID: 37122405 Free PMC article. No abstract available.
References
-
- Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, ... Spreafico R (2013). International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A task force report from the ILAE commission on diagnostic methods. Epilepsia, 54(7), 1315–1329. 10.1111/epi.12220 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

