Environmental and intrinsic modulations of venous differentiation
- PMID: 35987946
- PMCID: PMC11072674
- DOI: 10.1007/s00018-022-04470-4
Environmental and intrinsic modulations of venous differentiation
Abstract
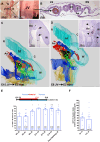
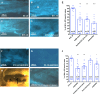
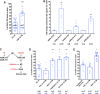
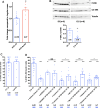
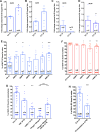
Endothelial cells in veins differ in morphology, function and gene expression from those in arteries and lymphatics. Understanding how venous and arterial identities are induced during development is required to understand how arterio-venous malformations occur, and to improve the outcome of vein grafts in surgery by promoting arterialization of veins. To identify factors that promote venous endothelial cell fate in vivo, we isolated veins from quail embryos, at different developmental stages, that were grafted into the coelom of chick embryos. Endothelial cells migrated out from the grafted vein and their colonization of host veins and/or arteries was quantified. We show that venous fate is promoted by sympathetic vessel innervation at embryonic day 11. Removal of sympathetic innervation decreased vein colonization, while norepinephrine enhanced venous colonization. BMP treatment or inhibition of ERK enhanced venous fate, revealing environmental neurotransmitter and BMP signaling and intrinsic ERK inhibition as actors in venous fate acquisition. We also identify the BMP antagonist Noggin as a potent mediator of venous arterialization.
Keywords: Avian embryo; BMPs; ERK; Sympathetic pathway; Venous differentiation.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
References
-
- Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779. - PubMed
-
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

