Surveying nonvisual arrestins reveals allosteric interactions between functional sites
- PMID: 35988049
- PMCID: PMC9771995
- DOI: 10.1002/prot.26413
Surveying nonvisual arrestins reveals allosteric interactions between functional sites
Abstract
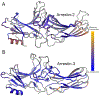
Arrestins are important scaffolding proteins that are expressed in all vertebrate animals. They regulate cell-signaling events upon binding to active G-protein coupled receptors (GPCR) and trigger endocytosis of active GPCRs. While many of the functional sites on arrestins have been characterized, the question of how these sites interact is unanswered. We used anisotropic network modeling (ANM) together with our covariance compliment techniques to survey all the available structures of the nonvisual arrestins to map how structural changes and protein-binding affect their structural dynamics. We found that activation and clathrin binding have a marked effect on arrestin dynamics, and that these dynamics changes are localized to a small number of distant functional sites. These sites include α-helix 1, the lariat loop, nuclear localization domain, and the C-domain β-sheets on the C-loop side. Our techniques suggest that clathrin binding and/or GPCR activation of arrestin perturb the dynamics of these sites independent of structural changes.
Keywords: GPCR; allostery; anisotropic network modeling; arrestin.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Statements and Declarations
The authors have no financial or non-financial interests to declare
Figures




Similar articles
-
β-arrestins and G protein-coupled receptor trafficking.Handb Exp Pharmacol. 2014;219:173-86. doi: 10.1007/978-3-642-41199-1_9. Handb Exp Pharmacol. 2014. PMID: 24292830 Free PMC article. Review.
-
β-Arrestins and G protein-coupled receptor trafficking.Methods Enzymol. 2013;521:91-108. doi: 10.1016/B978-0-12-391862-8.00005-3. Methods Enzymol. 2013. PMID: 23351735 Review.
-
Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between beta-arrestins and AP-2.J Biol Chem. 2007 Oct 5;282(40):29089-100. doi: 10.1074/jbc.M700577200. Epub 2007 Aug 3. J Biol Chem. 2007. PMID: 17675294
-
β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation.Nat Cell Biol. 2016 Mar;18(3):303-10. doi: 10.1038/ncb3307. Epub 2016 Feb 1. Nat Cell Biol. 2016. PMID: 26829388 Free PMC article.
-
Arrestin-Dependent and -Independent Internalization of G Protein-Coupled Receptors: Methods, Mechanisms, and Implications on Cell Signaling.Mol Pharmacol. 2021 Apr;99(4):242-255. doi: 10.1124/molpharm.120.000192. Epub 2021 Jan 20. Mol Pharmacol. 2021. PMID: 33472843 Review.
Cited by
-
Association of Neurokinin-1 Receptor Signaling Pathways with Cancer.Curr Med Chem. 2024;31(39):6460-6486. doi: 10.2174/0929867331666230818110812. Curr Med Chem. 2024. PMID: 37594106 Review.
-
Membrane phosphoinositides allosterically tune β-arrestin dynamics to facilitate GPCR core engagement.bioRxiv [Preprint]. 2025 Jul 23:2025.06.06.658200. doi: 10.1101/2025.06.06.658200. bioRxiv. 2025. PMID: 40501946 Free PMC article. Preprint.
References
-
- Goldsmith ZG and Dhanasekaran DN, G protein regulation of MAPK networks. Oncogene, 2007. 26(22): p. 3122–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

