Promising use of immune cell-derived exosomes in the treatment of SARS-CoV-2 infections
- PMID: 35988156
- PMCID: PMC9393056
- DOI: 10.1002/ctm2.1026
Promising use of immune cell-derived exosomes in the treatment of SARS-CoV-2 infections
Abstract
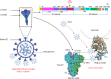
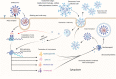
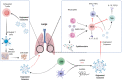
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is persistently threatening the lives of thousands of individuals globally. It triggers pulmonary oedema, driving to dyspnoea and lung failure. Viral infectivity of coronavirus disease 2019 (COVID-19) is a genuine challenge due to the mutagenic genome and mysterious immune-pathophysiology. Early reports highlighted that extracellular vesicles (exosomes, Exos) work to enhance COVID-19 progression by mediating viral transmission, replication and mutations. Furthermore, recent studies revealed that Exos derived from immune cells play an essential role in the promotion of immune cell exhaustion by transferring regulatory lncRNAs and miRNAs from exhausted cells to the active cells. Fortunately, there are great chances to modulate the immune functions of Exos towards a sustained repression of COVID-19. Engineered Exos hold promising immunotherapeutic opportunities for remodelling cytotoxic T cells' function. Immune cell-derived Exos may trigger a stable epigenetic repression of viral infectivity, restore functional cytokine-producing T cells and rebalance immune response in severe infections by inducing functional T regulatory cells (Tregs). This review introduces a view on the current outcomes of immunopathology, and immunotherapeutic applications of immune cell-derived Exos in COVID-19, besides new perspectives to develop novel patterns of engineered Exos triggering novel anti-SARS-CoV-2 immune responses.
Keywords: SARS-CoV-2; epigenetics; exhaustion; exosomes; immunotherapy.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare there are no financial or non‐financial conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
