Effect of intra-partum azithromycin on the development of the infant nasopharyngeal microbiota: A post hoc analysis of a double-blind randomized trial
- PMID: 35988464
- PMCID: PMC9420482
- DOI: 10.1016/j.ebiom.2022.104227
Effect of intra-partum azithromycin on the development of the infant nasopharyngeal microbiota: A post hoc analysis of a double-blind randomized trial
Abstract
Background: Sepsis is a leading cause of neonatal death. Intrapartum azithromycin reduces neonatal nasopharyngeal carriage of potentially pathogenic bacteria, a prerequisite for sepsis. Early antibiotic exposure has been associated with microbiota perturbations with varying effects. This study aims to understand the effect of intrapartum azithromycin intervention on the developing nasopharyngeal microbiota of the child.
Methods: Using 16S rRNA gene sequencing, we analysed the microbiota of 343 nasopharyngeal samples collected from birth to 12 months from 109 healthy infants selected from a double-blind randomized placebo-controlled clinical trial conducted in the Gambia (PregnAnZI-1). In the trial, 829 women were given 2g oral azithromycin or placebo (1:1) during labour with the objective of reducing bacterial carriage in mother and child during the neonatal period. The post-hoc analysis presented here assessed the effect of the intervention on the child nasopharyngeal microbiota development.
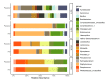
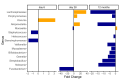
Findings: 55 children were from mothers given azithromycin and 54 from mothers given placebo. Comparing arms, we found an increase in alpha-diversity at day-6 (p = 0·018), and a significant effect on overall microbiota composition at days 6 and 28 (R2 = 4.4%, q = 0·007 and R2 = 2.3%, q = 0·018 respectively). At genus level, we found lower representation of Staphylococcus at day-6 (q = 0·0303) and higher representation of Moraxella at 12 months (q = 0·0443). Unsupervised clustering of samples by microbial community similarity showed different community dynamics between the intervention and placebo arms during the neonatal period.
Interpretation: These results indicate that intrapartum azithromycin caused short-term alterations in the nasopharyngeal microbiota with modest overall effect at 12 months of age. Further exploration of the effects of these variations on microbiome function will give more insight on the potential risks and benefits, for the child, associated with this intervention.
Funding: This work was jointly funded by the Medical Research Council (UK) (MC_EX_MR/J010391/1/MRC), Bill & Melinda Gates Foundation (OPP1196513), and MRCG@LSHTM Doctoral Training Program.
Keywords: Azithromycin; Infant; Intrapartum; Nasopharyngeal microbiota; West Africa.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no conflicting interests.
Figures




Similar articles
-
Effect of intrapartum azithromycin on gut microbiota development in early childhood: A post hoc analysis of a double-blind randomized trial.iScience. 2024 Aug 3;27(9):110626. doi: 10.1016/j.isci.2024.110626. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262807 Free PMC article.
-
Oral azithromycin given during labour decreases bacterial carriage in the mothers and their offspring: a double-blind randomized trial.Clin Microbiol Infect. 2016 Jun;22(6):565.e1-9. doi: 10.1016/j.cmi.2016.03.005. Epub 2016 Mar 26. Clin Microbiol Infect. 2016. PMID: 27026482 Free PMC article. Clinical Trial.
-
Impact of Intrapartum Azithromycin on the Carriage and Antibiotic Resistance of Escherichia coli and Klebsiella pneumoniae in Mothers and Their Newborns: A Substudy of a Randomized, Double-Blind Trial Conducted in The Gambia and Burkina Faso.Clin Infect Dis. 2024 Dec 17;79(6):1338-1345. doi: 10.1093/cid/ciae280. Clin Infect Dis. 2024. PMID: 38752311 Free PMC article. Clinical Trial.
-
Impact of intra-partum azithromycin on carriage of group A streptococcus in the Gambia: a posthoc analysis of a double-blind randomized placebo-controlled trial.BMC Infect Dis. 2022 Jan 29;22(1):103. doi: 10.1186/s12879-022-07080-4. BMC Infect Dis. 2022. PMID: 35093029 Free PMC article. Clinical Trial.
-
Azithromycin Exposure Induces Transient Microbial Composition Shifts and Decreases the Airway Microbiota Resilience from Outdoor PM2.5 Stress in Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled Trial.Microbiol Spectr. 2023 Jun 15;11(3):e0206622. doi: 10.1128/spectrum.02066-22. Epub 2023 Apr 24. Microbiol Spectr. 2023. PMID: 37093053 Free PMC article. Clinical Trial.
Cited by
-
Current state of microbiota clinical applications in neonatal and pediatric bacterial infections.Gut Microbes. 2025 Dec;17(1):2529400. doi: 10.1080/19490976.2025.2529400. Epub 2025 Jul 6. Gut Microbes. 2025. PMID: 40618377 Free PMC article. Review.
-
Effect of intrapartum azithromycin on gut microbiota development in early childhood: A post hoc analysis of a double-blind randomized trial.iScience. 2024 Aug 3;27(9):110626. doi: 10.1016/j.isci.2024.110626. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262807 Free PMC article.
-
Effect of intrapartum azithromycin on early childhood gut mycobiota development: post hoc analysis of a double-blind randomized trial.Nat Commun. 2025 Aug 9;16(1):7356. doi: 10.1038/s41467-025-62142-w. Nat Commun. 2025. PMID: 40783383 Free PMC article. Clinical Trial.
References
-
- WHO . World Heal Organ; 2019. Newborns: Reducing Mortality.https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-morta... Accessed 1 July 2020.
-
- WHO . World Heal Organ; 2017. WHO|SDG 3: Ensure Healthy Lives and Promote Wellbeing for all at all Ages.http://www.who.int/sdg/targets/en/ Accessed 1 July 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

