Maintenance on extended-release naltrexone is associated with reduced injection opioid use among justice-involved persons with opioid use disorder
- PMID: 35988513
- PMCID: PMC9509444
- DOI: 10.1016/j.jsat.2022.108852
Maintenance on extended-release naltrexone is associated with reduced injection opioid use among justice-involved persons with opioid use disorder
Erratum in
-
Corrigendum to 'Maintenance on extended-release naltrexone is associated with reduced injection opioid use among justice-involved persons with opioid use disorder' [J. Subst. Abuse Treat. vol. 142 (2022)/108852].J Subst Use Addict Treat. 2023 Jul;150:209074. doi: 10.1016/j.josat.2023.209074. Epub 2023 Jun 3. J Subst Use Addict Treat. 2023. PMID: 37271717 No abstract available.
Abstract
Introduction: Opioid use disorder (OUD) and injection drug use (IDU) place justice-involved individuals at increased risk for acquiring or transmitting HIV or hepatitis C virus (HCV). Methadone and buprenorphine have been associated with reduced opioid IDU; however, the effect of extended-release naltrexone (XR-NTX) on this behavior is incompletely studied.
Methods: This study examined injection opioid use and shared injection equipment behavior from a completed double-blind placebo-controlled trial of XR-NTX among 88 justice-involved participants with HIV and OUD. Changes in participants' self-reported daily injection opioid use and shared injection equipment was evaluated pre-incarceration, during incarceration, and monthly post-release for 6 months. The study also assessed differences in time to first opioid injection post-release. The research team performed intention to treat and "as treated" (high treatment versus low treatment) analyses.
Results: Fifty-eight of 88 participants (69.5 %) endorsed IDU and 26 (29.5 %) reported sharing injection equipment in the 30 days pre-incarceration; 2 participants (2.2 %) reported IDU during incarceration; 19 (21.6 %) reported IDU one month post-release from prison or jail. Fifty-four (61.4 %) participants had an HIV RNA below 200 copies/mL and 62 (70.5 %) were baseline HCV antibody positive. The 6-month follow-up rate was 49.5 % and 50.5 % for those who received XR-NTX and placebo, respectively, which was not significantly different (p = 0.822). Participants in the XR-NTX and placebo groups had similar low mean opioid injection use post-release and time to first injection opioid use in the Intention-to-treat analysis. In the as-treated analysis, participants in the high treatment group had significantly lower mean proportion of days injecting opioids (13.8 % high treatment versus 22.8 % low treatment, p = 0.02) by month 1, which persisted up to 5 months post-release (0 % high treatment vs 24.3 % low treatment, p < 0.001) and experienced a longer time to first opioid injection post-release (143.8 days high treatment vs 67.4 days low treatment, p < 0.001).
Conclusions: Injection opioid use was low during incarceration and remained low post-release in this justice-involved population. Retention on XR-NTX was associated with reduced intravenous opioid use, which has important implications for reducing transmission of HIV and HCV.
Keywords: Extended-release naltrexone; HIV; Hepatitis C virus; Injection drug use; Medications for opioid use disorder; Opioid use disorder.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest Author SAS has provided scientific consultation to Alkermes Inc. and received NIH and VA grant funding. SAS has received in-kind study drug donations from Alkermes Inc. and Indivior Pharmaceutical Company for NIH-funded research. The authors alone are responsible for the content and writing of this paper.
Figures
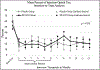
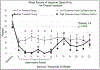


Similar articles
-
HIV clinic-based extended-release naltrexone versus treatment as usual for people with HIV and opioid use disorder: a non-blinded, randomized non-inferiority trial.Addiction. 2022 Jul;117(7):1961-1971. doi: 10.1111/add.15836. Epub 2022 Mar 2. Addiction. 2022. PMID: 35129242 Free PMC article. Clinical Trial.
-
Extended-release naltrexone for youth with opioid use disorder.J Subst Abuse Treat. 2021 Nov;130:108407. doi: 10.1016/j.jsat.2021.108407. Epub 2021 Apr 15. J Subst Abuse Treat. 2021. PMID: 34118699 Free PMC article. Clinical Trial.
-
Perceptions of extended-release naltrexone, methadone, and buprenorphine treatments following release from jail.Addict Sci Clin Pract. 2019 Oct 1;14(1):37. doi: 10.1186/s13722-019-0166-0. Addict Sci Clin Pract. 2019. PMID: 31570100 Free PMC article.
-
Extended-release injectable naltrexone for opioid use disorder: a systematic review.Addiction. 2018 Jul;113(7):1188-1209. doi: 10.1111/add.14180. Epub 2018 Mar 24. Addiction. 2018. PMID: 29396985 Free PMC article.
-
Acceptability and efficacy of naltrexone for criminal justice-involved individuals with opioid use disorder: a systematic review and meta-analysis.Addiction. 2020 Aug;115(8):1413-1425. doi: 10.1111/add.14946. Epub 2020 Jan 17. Addiction. 2020. PMID: 31863669
Cited by
-
HIV and Substance Use Disorders.Infect Dis Clin North Am. 2024 Sep;38(3):599-611. doi: 10.1016/j.idc.2024.06.003. Epub 2024 Jul 2. Infect Dis Clin North Am. 2024. PMID: 38960783 Review.
-
Extended-release pharmacotherapies for substance use disorders in incarcerated populations: A systematic review.Addiction. 2025 May;120(5):835-859. doi: 10.1111/add.16766. Epub 2025 Jan 30. Addiction. 2025. PMID: 39888117 Free PMC article.
-
The relationship between reincarceration and treatment of opioid use disorder with extended-release naltrexone among persons with HIV.Drug Alcohol Depend Rep. 2023 Apr 14;7:100159. doi: 10.1016/j.dadr.2023.100159. eCollection 2023 Jun. Drug Alcohol Depend Rep. 2023. PMID: 37159815 Free PMC article.
-
Considerations when prescribing opioid agonist therapies for people living with HIV.Expert Rev Clin Pharmacol. 2024 Jul;17(7):549-564. doi: 10.1080/17512433.2024.2375448. Epub 2024 Jul 4. Expert Rev Clin Pharmacol. 2024. PMID: 38946101 Free PMC article. Review.
References
-
- Ahmad FB, Anderson RN, Cisewski JA, Rossen LM, Warner M, & Sutton P (2021, November 4). Provisional county-level drug overdose death counts. Retrieved January 1, 2022, from https://www.cdc.gov/nchs/nvss/vsrr/prov-county-drug-overdose.htm.
-
- Akiyama MJ, Lipsey D, Heo M, Agyemang L, Norton BL, Hidalgo J, Lora K, & Litwin AH (2020). Low hepatitis C reinfection following direct-acting antiviral therapy among people who inject drugs on opioid agonist therapy. Clinical Infectious Diseases, 70(12), 2695–2702. 10.1093/cid/ciz693 - DOI - PMC - PubMed
-
- Biondi BE, Mohanty S, Wyk BV, Montgomery RR, Shaw AC, & Springer SA (2021). Design and implementation of a prospective cohort study of persons living with and without HIV infection who are initiating medication treatment for opioid use disorder. Contemporary Clinical Trials Communications, 21, Article 100704. 10.1016/j.conctc.2021.100704 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

