Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study
- PMID: 35988572
- PMCID: PMC9388060
- DOI: 10.1016/S2213-2600(22)00226-0
Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study
Erratum in
-
Correction to Lancet Respir Med 2022; published online Aug 18. https://doi.org/10.1016/S2213-2600(22)00226-0.Lancet Respir Med. 2022 Nov;10(11):e106. doi: 10.1016/S2213-2600(22)00362-9. Epub 2022 Sep 5. Lancet Respir Med. 2022. PMID: 36075240 Free PMC article. No abstract available.
Abstract
Background: Knowledge of the window of SARS-CoV-2 infectiousness is crucial in developing policies to curb transmission. Mathematical modelling based on scarce empirical evidence and key assumptions has driven isolation and testing policy, but real-world data are needed. We aimed to characterise infectiousness across the full course of infection in a real-world community setting.
Methods: The Assessment of Transmission and Contagiousness of COVID-19 in Contacts (ATACCC) study was a UK prospective, longitudinal, community cohort of contacts of newly diagnosed, PCR-confirmed SARS-CoV-2 index cases. Household and non-household exposed contacts aged 5 years or older were eligible for recruitment if they could provide informed consent and agree to self-swabbing of the upper respiratory tract. The primary objective was to define the window of SARS-CoV-2 infectiousness and its temporal correlation with symptom onset. We quantified viral RNA load by RT-PCR and infectious viral shedding by enumerating cultivable virus daily across the course of infection. Participants completed a daily diary to track the emergence of symptoms. Outcomes were assessed with empirical data and a phenomenological Bayesian hierarchical model.
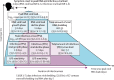
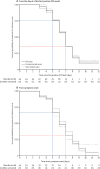
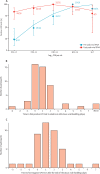
Findings: Between Sept 13, 2020, and March 31, 2021, we enrolled 393 contacts from 327 households (the SARS-CoV-2 pre-alpha and alpha variant waves); and between May 24, 2021, and Oct 28, 2021, we enrolled 345 contacts from 215 households (the delta variant wave). 173 of these 738 contacts were PCR positive for more than one timepoint, 57 of which were at the start of infection and comprised the final study population. The onset and end of infectious viral shedding were captured in 42 cases and the median duration of infectiousness was 5 (IQR 3-7) days. Although 24 (63%) of 38 cases had PCR-detectable virus before symptom onset, only seven (20%) of 35 shed infectious virus presymptomatically. Symptom onset was a median of 3 days before both peak viral RNA and peak infectious viral load (viral RNA IQR 3-5 days, n=38; plaque-forming units IQR 3-6 days, n=35). Notably, 22 (65%) of 34 cases and eight (24%) of 34 cases continued to shed infectious virus 5 days and 7 days post-symptom onset, respectively (survival probabilities 67% and 35%). Correlation of lateral flow device (LFD) results with infectious viral shedding was poor during the viral growth phase (sensitivity 67% [95% CI 59-75]), but high during the decline phase (92% [86-96]). Infectious virus kinetic modelling suggested that the initial rate of viral replication determines the course of infection and infectiousness.
Interpretation: Less than a quarter of COVID-19 cases shed infectious virus before symptom onset; under a crude 5-day self-isolation period from symptom onset, two-thirds of cases released into the community would still be infectious, but with reduced infectious viral shedding. Our findings support a role for LFDs to safely accelerate deisolation but not for early diagnosis, unless used daily. These high-resolution, community-based data provide evidence to inform infection control guidance.
Funding: National Institute for Health and Care Research.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NF reports grants from the UK Medical Research Council, UK National Institute of Health and Care Research (NIHR), UK Research and Innovation, Community Jameel, Janssen Pharmaceuticals, Bill & Melinda Gates Foundation, and Gavi, the Vaccine Alliance; consulting fees from the World Bank; payment or honoraria from the Wellcome Trust; travel expenses from WHO; advisory board participation for Takeda; and is a senior editor of the eLife journal. All other authors declare no competing interests.
Figures


Comment in
-
SARS-CoV-2: can isolation be limited to those who are truly infectious?Lancet Respir Med. 2022 Nov;10(11):1011-1013. doi: 10.1016/S2213-2600(22)00272-7. Epub 2022 Oct 10. Lancet Respir Med. 2022. PMID: 36228636 Free PMC article. No abstract available.
References
-
- Killingley B, Mann AJ, Kalinova M, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28:1031–1041. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

