Peroxisomal regulation of energy homeostasis: Effect on obesity and related metabolic disorders
- PMID: 35988716
- PMCID: PMC9442330
- DOI: 10.1016/j.molmet.2022.101577
Peroxisomal regulation of energy homeostasis: Effect on obesity and related metabolic disorders
Abstract
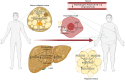
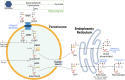
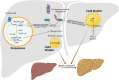
Background: Peroxisomes are single membrane-bound organelles named for their role in hydrogen peroxide production and catabolism. However, their cellular functions extend well beyond reactive oxygen species (ROS) metabolism and include fatty acid oxidation of unique substrates that cannot be catabolized in mitochondria, and synthesis of ether lipids and bile acids. Metabolic functions of peroxisomes involve crosstalk with other organelles, including mitochondria, endoplasmic reticulum, lipid droplets and lysosomes. Emerging studies suggest that peroxisomes are important regulators of energy homeostasis and that disruption of peroxisomal functions influences the risk for obesity and the associated metabolic disorders, including type 2 diabetes and hepatic steatosis.
Scope of review: Here, we focus on the role of peroxisomes in ether lipid synthesis, β-oxidation and ROS metabolism, given that these functions have been most widely studied and have physiologically relevant implications in systemic metabolism and obesity. Efforts are made to mechanistically link these cellular and systemic processes.
Major conclusions: Circulating plasmalogens, a form of ether lipids, have been identified as inversely correlated biomarkers of obesity. Ether lipids influence metabolic homeostasis through multiple mechanisms, including regulation of mitochondrial morphology and respiration affecting brown fat-mediated thermogenesis, and through regulation of adipose tissue development. Peroxisomal β-oxidation also affects metabolic homeostasis through generation of signaling molecules, such as acetyl-CoA and ROS that inhibit hydrolysis of stored lipids, contributing to development of hepatic steatosis. Oxidative stress resulting from increased peroxisomal β-oxidation-generated ROS in the context of obesity mediates β-cell lipotoxicity. A better understanding of the roles peroxisomes play in regulating and responding to obesity and its complications will provide new opportunities for their treatment.
Keywords: Diabetes; Fatty liver; Lipid metabolism; Obesity; Peroxisomes; Plasmalogen.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures

Similar articles
-
Fatty Acid Oxidation in Peroxisomes: Enzymology, Metabolic Crosstalk with Other Organelles and Peroxisomal Disorders.Adv Exp Med Biol. 2020;1299:55-70. doi: 10.1007/978-3-030-60204-8_5. Adv Exp Med Biol. 2020. PMID: 33417207 Review.
-
Peroxisomes as cellular adaptors to metabolic and environmental stress.Trends Cell Biol. 2021 Aug;31(8):656-670. doi: 10.1016/j.tcb.2021.02.005. Epub 2021 Mar 2. Trends Cell Biol. 2021. PMID: 33674166 Free PMC article. Review.
-
[The contribution of peroxisomes to lipid metabolism].J Clin Chem Clin Biochem. 1986 Feb;24(2):109-18. J Clin Chem Clin Biochem. 1986. PMID: 3711795 German.
-
Targeting peroxisomal fatty acid oxidation improves hepatic steatosis and insulin resistance in obese mice.J Biol Chem. 2023 Feb;299(2):102845. doi: 10.1016/j.jbc.2022.102845. Epub 2022 Dec 28. J Biol Chem. 2023. PMID: 36586435 Free PMC article.
-
Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging.Antioxidants (Basel). 2022 Jan 19;11(2):192. doi: 10.3390/antiox11020192. Antioxidants (Basel). 2022. PMID: 35204075 Free PMC article. Review.
Cited by
-
Quantitative Lipid Profiling Reveals Major Differences between Liver Organoids with Normal Pi*M and Deficient Pi*Z Variants of Alpha-1-antitrypsin.Int J Mol Sci. 2023 Aug 5;24(15):12472. doi: 10.3390/ijms241512472. Int J Mol Sci. 2023. PMID: 37569847 Free PMC article.
-
Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension.Int J Mol Sci. 2025 Jun 3;26(11):5355. doi: 10.3390/ijms26115355. Int J Mol Sci. 2025. PMID: 40508164 Free PMC article. Review.
-
The Effect of Omega-3 on Mitigating Exercise-Induced Muscle Damage.Cureus. 2025 Apr 1;17(4):e81559. doi: 10.7759/cureus.81559. eCollection 2025 Apr. Cureus. 2025. PMID: 40313441 Free PMC article. Review.
-
Regulation of Mitochondrial and Peroxisomal Metabolism in Female Obesity and Type 2 Diabetes.Int J Mol Sci. 2024 Oct 19;25(20):11237. doi: 10.3390/ijms252011237. Int J Mol Sci. 2024. PMID: 39457018 Free PMC article. Review.
-
Dietary lipid is largely deposited in skin and rapidly affects insulating properties.Res Sq [Preprint]. 2024 Oct 7:rs.3.rs-3957002. doi: 10.21203/rs.3.rs-3957002/v2. Res Sq. 2024. Update in: Nat Commun. 2025 May 16;16(1):4570. doi: 10.1038/s41467-025-59869-x. PMID: 38464106 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

