Carboxypeptidase E and its splice variants: Key regulators of growth and metastasis in multiple cancer types
- PMID: 35988818
- PMCID: PMC9532369
- DOI: 10.1016/j.canlet.2022.215882
Carboxypeptidase E and its splice variants: Key regulators of growth and metastasis in multiple cancer types
Abstract
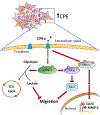
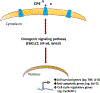
Mechanisms driving tumor growth and metastasis are complex, and involve the recruitment of many genes working in concert with each other. The tumor is characterized by the expression of specific sets of genes depending on its environment. Here we review the role of the carboxypeptidase E (CPE) gene which has been shown to be important in driving growth, survival and metastasis in many cancer types. CPE was first discovered as a prohormone processing enzyme, enriched in endocrine tumors, and later found to be expressed and secreted from many epithelial-derived tumors and cancer cell lines. Numerous studies have shown that besides wild-type CPE, a N-terminal truncated splice variant form of CPE (CPE-ΔN) has been cloned and found to be highly expressed in malignant tumors and cell lines derived from prostate, breast, liver and lung cancers and gliomas. The mechanisms of action of CPE and the splice variant in promoting tumor growth and metastasis in different cancer types are discussed. Mechanistically, secreted CPE activates the Erk/wnt pathways, while CPE-ΔN interacts with HDACs in a protein complex in the nucleus, to recruit various cell cycle genes and metastatic genes, respectively. Clinical studies suggest that CPE and CPE-ΔN mRNA and protein are potential diagnostic and prognostic biomarkers for multiple cancer types, assayed using solid tumors and secreted serum exosomes. CPE has been shown to be a therapeutic target for multiple cancer types. CPE/CPE-ΔN siRNA transported via exosomes and taken up by recipient high metastatic cancer cells, suppressed growth and proliferation of these cells. Thus future studies, delivering CPE/CPE-ΔN siRNA, perhaps via exosomes, to the tumor could be a novel treatment approach to suppress tumor growth and metastasis.
Keywords: Exosomes; Glioblastoma; Hepatocellular carcinoma; Lung adenocarcinoma; Osteosarcoma.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Exosomal Carboxypeptidase E (CPE) and CPE-shRNA-Loaded Exosomes Regulate Metastatic Phenotype of Tumor Cells.Int J Mol Sci. 2022 Mar 14;23(6):3113. doi: 10.3390/ijms23063113. Int J Mol Sci. 2022. PMID: 35328535 Free PMC article.
-
Carboxypeptidase E-ΔN promotes migration, invasiveness, and epithelial-mesenchymal transition of human osteosarcoma cells via the Wnt-β-catenin pathway.Biochem Cell Biol. 2019 Aug;97(4):446-453. doi: 10.1139/bcb-2018-0236. Epub 2018 Dec 3. Biochem Cell Biol. 2019. PMID: 30508384
-
An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers.J Clin Invest. 2011 Mar;121(3):880-92. doi: 10.1172/JCI40433. J Clin Invest. 2011. Retraction in: J Clin Invest. 2019 Mar 18;130:1804. doi: 10.1172/JCI128836. PMID: 21285511 Free PMC article. Retracted.
-
New roles of carboxypeptidase E in endocrine and neural function and cancer.Endocr Rev. 2012 Apr;33(2):216-53. doi: 10.1210/er.2011-1039. Epub 2012 Mar 7. Endocr Rev. 2012. PMID: 22402194 Free PMC article. Review.
-
Dissecting carboxypeptidase E: properties, functions and pathophysiological roles in disease.Endocr Connect. 2017 May;6(4):R18-R38. doi: 10.1530/EC-17-0020. Epub 2017 Mar 27. Endocr Connect. 2017. PMID: 28348001 Free PMC article. Review.
Cited by
-
GTSE1-expressed osteoblastic cells facilitate formation of pro-metastatic tumor microenvironment in osteosarcoma.Genes Dis. 2025 Mar 7;12(6):101591. doi: 10.1016/j.gendis.2025.101591. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40831537 Free PMC article.
-
Integrating multiomics analysis and machine learning to refine the molecular subtyping and prognostic analysis of stomach adenocarcinoma.Sci Rep. 2025 Jan 30;15(1):3843. doi: 10.1038/s41598-025-87444-3. Sci Rep. 2025. PMID: 39885324 Free PMC article.
-
Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment.Bone Res. 2023 Feb 27;11(1):11. doi: 10.1038/s41413-023-00246-z. Bone Res. 2023. PMID: 36849442 Free PMC article. Review.
-
Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis.Extracell Vesicles Circ Nucl Acids. 2023;4(4):568-587. doi: 10.20517/evcna.2023.34. Epub 2023 Nov 9. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 38435713 Free PMC article.
-
Carboxypeptidase E is a prognostic biomarker co-expressed with osteoblastic genes in osteosarcoma.PeerJ. 2023 Aug 30;11:e15814. doi: 10.7717/peerj.15814. eCollection 2023. PeerJ. 2023. PMID: 37663298 Free PMC article.
References
-
- Fricker LD, Snyder SH, Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase, J Biol Chem, 258 (1983) 10950–10955. - PubMed
-
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP, Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice, Cell, 88 (1997) 73–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

