A 3D Peptide/[60]Fullerene Hybrid for Multivalent Recognition
- PMID: 35989251
- PMCID: PMC9826239
- DOI: 10.1002/anie.202210043
A 3D Peptide/[60]Fullerene Hybrid for Multivalent Recognition
Abstract
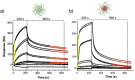
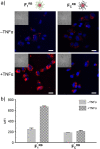
Fully substituted peptide/[60]fullerene hexakis-adducts offer an excellent opportunity for multivalent protein recognition. In contrast to monofunctionalized fullerene hybrids, peptide/[60]fullerene hexakis-adducts display multiple copies of a peptide in close spatial proximity and in the three dimensions of space. High affinity peptide binders for almost any target can be currently identified by in vitro evolution techniques, often providing synthetically simpler alternatives to natural ligands. However, despite the potential of peptide/[60]fullerene hexakis-adducts, these promising conjugates have not been reported to date. Here we present a synthetic strategy for the construction of 3D multivalent hybrids that are able to bind with high affinity the E-selectin. The here synthesized fully substituted peptide/[60]fullerene hybrids and their multivalent recognition of natural receptors constitute a proof of principle for their future application as functional biocompatible materials.
Keywords: Fullerenes; Glycomimetic; Lectin; Multivalency; Peptides.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Fullerene-sp2-iminosugar balls as multimodal ligands for lectins and glycosidases: a mechanistic hypothesis for the inhibitory multivalent effect.Chemistry. 2013 Dec 2;19(49):16791-803. doi: 10.1002/chem.201303158. Epub 2013 Oct 22. Chemistry. 2013. PMID: 24150869
-
Maleimide and Cyclooctyne-Based Hexakis-Adducts of Fullerene: Multivalent Scaffolds for Copper-Free Click Chemistry on Fullerenes.J Org Chem. 2018 Feb 16;83(4):1727-1736. doi: 10.1021/acs.joc.7b02402. Epub 2018 Feb 2. J Org Chem. 2018. PMID: 29310437
-
Potent Glycosidase Inhibition with Heterovalent Fullerenes: Unveiling the Binding Modes Triggering Multivalent Inhibition.Chemistry. 2016 Aug 1;22(32):11450-60. doi: 10.1002/chem.201601673. Epub 2016 Jul 4. Chemistry. 2016. PMID: 27374430
-
Synthesis and biological application of glyco- and peptide derivatives of fullerene C60.Eur J Med Chem. 2022 Feb 15;230:114104. doi: 10.1016/j.ejmech.2022.114104. Epub 2022 Jan 10. Eur J Med Chem. 2022. PMID: 35051749 Review.
-
Fullerene sugar balls: a new class of biologically active fullerene derivatives.Chem Asian J. 2014 Jun;9(6):1436-44. doi: 10.1002/asia.201400133. Epub 2014 Mar 26. Chem Asian J. 2014. PMID: 24678063 Review.
Cited by
-
Synthesis of [60]Fullerene Hybrids Endowed with Steroids and Monosaccharides: Theoretical Underpinning as Promising anti-SARS-CoV-2 Agents.European J Org Chem. 2023 Jan 18:e202201301. doi: 10.1002/ejoc.202201301. Online ahead of print. European J Org Chem. 2023. PMID: 36721524 Free PMC article.
-
Polyvalent Glycomimetic-Gold Nanoparticles Revealing Critical Roles of Glycan Display on Multivalent Lectin-Glycan Interaction Biophysics and Antiviral Properties.JACS Au. 2024 Aug 15;4(8):3295-3309. doi: 10.1021/jacsau.4c00610. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211605 Free PMC article.
-
Carbon-based glyco-nanoplatforms: towards the next generation of glycan-based multivalent probes.Chem Soc Rev. 2022 Dec 12;51(24):9960-9985. doi: 10.1039/d2cs00741j. Chem Soc Rev. 2022. PMID: 36416290 Free PMC article. Review.
References
-
- Lauster D., Klenk S., Ludwig K., Nojoumi S., Behren S., Adam L., Stadtmüller M., Saenger S., Zimmler S., Hönzke K., et al., Nat. Nanotechnol. 2020, 15, 373–379. - PubMed
-
- Gallego I., Rioboo A., Reina J. J., Díaz B., Canales Á., Cañada F. J., Guerra-Varela J., Sánchez L., Montenegro J., ChemBioChem 2019, 20, 1400–1409. - PubMed
-
- Juanes M., Lostalé-Seijo I., Granja J. R., Montenegro J., Chem. Eur. J. 2018, 24, 10689–10698. - PubMed
-
- Kwon S. J., Na D. H., Kwak J. H., Douaisi M., Zhang F., Park E. J., Park J. H., Youn H., Song C. S., Kane R. S., et al., Nat. Nanotechnol. 2017, 12, 48–54. - PubMed

