Association of Systemic Adverse Reactions and Serum SARS-CoV-2 Spike Protein Antibody Levels after Administration of BNT162b2 mRNA COVID-19 Vaccine
- PMID: 35989281
- PMCID: PMC9683821
- DOI: 10.2169/internalmedicine.9699-22
Association of Systemic Adverse Reactions and Serum SARS-CoV-2 Spike Protein Antibody Levels after Administration of BNT162b2 mRNA COVID-19 Vaccine
Abstract
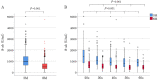
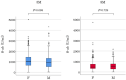
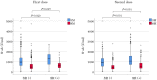
Objectives The influential factors for anti-severe acute respiratory syndrome coronavirus 2 spike protein antibody (S-ab) levels were assessed after the administration of BNT162b2 mRNA coronavirus disease-2019 (COVID-19) vaccine at short and medium terms. Methods A total of 470 healthcare workers (118 males, mean age 41.0±11.9 years) underwent serum S-ab level measurement at 3 and 8 months after two inoculations of BNT162b2 vaccine given 3 weeks apart, who had no history of COVID-19 were enrolled in this study. The changes and differences after vaccination due to gender and adverse reactions of S-ab were analyzed. Results Systemic adverse reactions incidence (48%) was significantly higher after the second dose than after the first dose (8%). S-ab levels decreased as the age increased (from the 20s to 60s) in both measurements. S-ab level 8 months after the second inoculation [median 476.3 (interquartile range (IQR) 322.4-750.6) U/mL] was significantly lower than that after 3 months [977.5 (637.2-1,409.0) U/mL; p<0.001]. The median decrease rate of S-ab levels in 5 months was 50.3% (IQR 40.3-62.6) and those differences were not observed among all generations. Gender-associated differences in S-ab levels were not observed; however, a significant relationship between higher S-ab levels and the systemic adverse reactions was observed at both measurements. Conclusions The systemic adverse reaction is an independent factor for higher S-ab levels at short and medium terms after BNT162b2 vaccination as demonstrated in our data.
Keywords: BNT162b2 mRNA COVID-19 vaccine; COVID-19; anti-SARS-CoV-2 spike protein antibody.
Conflict of interest statement
Figures
Similar articles
-
Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine.Front Immunol. 2022 Jan 31;13:827306. doi: 10.3389/fimmu.2022.827306. eCollection 2022. Front Immunol. 2022. PMID: 35173736 Free PMC article.
-
The association between adverse reactions and immune response against SARS-CoV-2 spike protein after vaccination with BNT162b2 among healthcare workers in a single healthcare system: a prospective observational cohort study.Hum Vaccin Immunother. 2022 Nov 30;18(5):2048559. doi: 10.1080/21645515.2022.2048559. Epub 2022 Mar 25. Hum Vaccin Immunother. 2022. PMID: 35333697 Free PMC article.
-
Factors associated with anti-SARS-CoV-2 spike antibody titers after a second BNT162b2 mRNA COVID-19 vaccination in Japanese hemodialysis patients.Clin Exp Nephrol. 2022 Sep;26(9):925-932. doi: 10.1007/s10157-022-02223-y. Epub 2022 Apr 15. Clin Exp Nephrol. 2022. PMID: 35426594 Free PMC article.
-
A Novel Development of Sarcoidosis Following COVID-19 Vaccination and a Literature Review.Intern Med. 2022 Oct 15;61(20):3101-3106. doi: 10.2169/internalmedicine.0104-22. Epub 2022 Aug 10. Intern Med. 2022. PMID: 35945009 Free PMC article. Review.
-
CVSARRP: A framework to predict the risk of adverse to severe adverse reactions for 10855 diseases after COVID-19 vaccination.Heliyon. 2023 Apr;9(4):e14828. doi: 10.1016/j.heliyon.2023.e14828. Epub 2023 Mar 27. Heliyon. 2023. PMID: 37009244 Free PMC article. Review.
Cited by
-
Autoantibodies Targeting G-Protein-Coupled Receptors and RAS-Related Molecules in Post-Acute COVID Vaccination Syndrome: A Retrospective Case Series Study.Biomedicines. 2024 Dec 15;12(12):2852. doi: 10.3390/biomedicines12122852. Biomedicines. 2024. PMID: 39767757 Free PMC article.
-
Serological response 5 months after the BNT162b2 COVID-19 vaccination in patients with various hematological disorders in Japan.Clin Exp Vaccine Res. 2023 Oct;12(4):319-327. doi: 10.7774/cevr.2023.12.4.319. Epub 2023 Oct 31. Clin Exp Vaccine Res. 2023. PMID: 38025915 Free PMC article.
-
Predictive Value of Reactogenicity for Anti-SARS-CoV-2 Antibody Response in mRNA-1273 Recipients: A Multicenter Prospective Cohort Study.Vaccines (Basel). 2023 Jan 3;11(1):120. doi: 10.3390/vaccines11010120. Vaccines (Basel). 2023. PMID: 36679965 Free PMC article.
-
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis.Front Public Health. 2024 Jul 30;12:1406315. doi: 10.3389/fpubh.2024.1406315. eCollection 2024. Front Public Health. 2024. PMID: 39139673 Free PMC article.
References
-
- Prime minister of Japan and His cabinet. Information related to COVID-19 [Internet]. Available from: https://japan.kantei.go.jp/ongoingtopics/vaccine.html
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

