The potential of predictive and prognostic breast MRI (P2-bMRI)
- PMID: 35989400
- PMCID: PMC9393116
- DOI: 10.1186/s41747-022-00291-z
The potential of predictive and prognostic breast MRI (P2-bMRI)
Abstract
Magnetic resonance imaging (MRI) is an important part of breast cancer diagnosis and multimodal workup. It provides unsurpassed soft tissue contrast to analyse the underlying pathophysiology, and it is adopted for a variety of clinical indications. Predictive and prognostic breast MRI (P2-bMRI) is an emerging application next to these indications. The general objective of P2-bMRI is to provide predictive and/or prognostic biomarkers in order to support personalisation of breast cancer treatment. We believe P2-bMRI has a great clinical potential, thanks to the in vivo examination of the whole tumour and of the surrounding tissue, establishing a link between pathophysiology and response to therapy (prediction) as well as patient outcome (prognostication). The tools used for P2-bMRI cover a wide spectrum: standard and advanced multiparametric pulse sequences; structured reporting criteria (for instance BI-RADS descriptors); artificial intelligence methods, including machine learning (with emphasis on radiomics data analysis); and deep learning that have shown compelling potential for this purpose. P2-bMRI reuses the imaging data of examinations performed in the current practice. Accordingly, P2-bMRI could optimise clinical workflow, enabling cost savings and ultimately improving personalisation of treatment. This review introduces the concept of P2-bMRI, focusing on the clinical application of P2-bMRI by using semantic criteria.
Keywords: Biomarkers; Breast neoplasms; Magnetic resonance imaging; Precision medicine; Prognosis.
© 2022. The Author(s) under exclusive licence to European Society of Radiology.
Conflict of interest statement
Matthias Dietzel and Pascal Baltzer are members of the
Figures

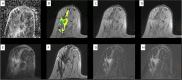
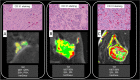
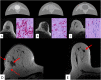


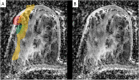
References
-
- Baltzer PAT, Krug KB, Dietzel M (2022) Evidence-Based and Structured Diagnosis in Breast MRI using the Kaiser Score. Rofo. 10.1055/a-1829-5985. - PubMed
