Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation
- PMID: 35989543
- PMCID: PMC9826214
- DOI: 10.1111/jce.15657
Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation
Abstract
Introduction: Multiple groups have reported on the usefulness of ablating in atrial regions exhibiting abnormal electrograms during atrial fibrillation (AF). Still, previous studies have suggested that ablation outcomes are highly operator- and center-dependent. This study sought to evaluate a novel machine learning software algorithm named VX1 (Volta Medical), trained to adjudicate multipolar electrogram dispersion.
Methods: This study was a prospective, multicentric, nonrandomized study conducted to assess the feasibility of generating VX1 dispersion maps. In 85 patients, 8 centers, and 17 operators, we compared the acute and long-term outcomes after ablation in regions exhibiting dispersion between primary and satellite centers. We also compared outcomes to a control group in which dispersion-guided ablation was performed visually by trained operators.
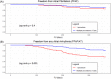
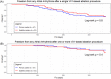
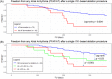
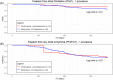
Results: The study population included 29% of long-standing persistent AF. AF termination occurred in 92% and 83% of the patients in primary and satellite centers, respectively, p = 0.31. The average rate of freedom from documented AF, with or without antiarrhythmic drugs (AADs), was 86% after a single procedure, and 89% after an average of 1.3 procedures per patient (p = 0.4). The rate of freedom from any documented atrial arrhythmia, with or without AADs, was 54% and 73% after a single or an average of 1.3 procedures per patient, respectively (p < 0.001). No statistically significant differences between outcomes of the primary versus satellite centers were observed for one (p = 0.8) or multiple procedures (p = 0.4), or between outcomes of the entire study population versus the control group (p > 0.2). Interestingly, intraprocedural AF termination and type of recurrent arrhythmia (i.e., AF vs. AT) appear to be predictors of the subsequent clinical course.
Conclusion: VX1, an expertise-based artificial intelligence software solution, allowed for robust center-to-center standardization of acute and long-term ablation outcomes after electrogram-based ablation.
Keywords: artificial intelligence; atrial fibrillation; catheter ablation; dispersion; driver; mapping; sinus rhythm.
© The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Figures

Comment in
-
Electrogram-based AF ablation: finally, reproducibility!J Cardiovasc Electrophysiol. 2022 Nov;33(11):2261-2262. doi: 10.1111/jce.15660. Epub 2022 Sep 18. J Cardiovasc Electrophysiol. 2022. PMID: 35989539 No abstract available.
References
-
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044‐2053. - PubMed
-
- O'Neill MD, Jaïs P, Takahashi Y, et al. The stepwise ablation approach for chronic atrial fibrillation—evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153‐167. - PubMed
-
- Estner HL, Hessling G, Biegler R, et al. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation—a prospective randomized study. Pacing Clin Electrophysiol. 2011;34:939‐948. - PubMed
-
- Seitz J, Horvilleur J, Curel L, et al. Active or passive pulmonary vein in atrial fibrillation: is pulmonary vein isolation always essential? Heart Rhythm. 2014;11:579‐586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

