Relapse Mechanism and Treatment Strategy After Chimeric Antigen Receptor T-Cell Therapy in Treating B-Cell Hematological Malignancies
- PMID: 35989682
- PMCID: PMC9403467
- DOI: 10.1177/15330338221118413
Relapse Mechanism and Treatment Strategy After Chimeric Antigen Receptor T-Cell Therapy in Treating B-Cell Hematological Malignancies
Abstract
Over the past few decades, immunotherapy has revolutionized the modern medical oncology field. Chimeric antigen receptor (CAR)-T cell therapy has a promising curative effect in the treatment of hematological malignancies. Anti-CD19 CAR-T cells are the most mature CAR-T cells recently studied and in recent years it has achieved a complete remission rate of approximately 90% in the treatment of B-cell acute lymphoblastic leukemia (B-ALL). Although CAR-T cell therapy has greatly alleviated the disease in patients with leukemia or lymphoma, some of them still relapse after treatment. Therefore, in this article, we discuss the factors that may contribute to disease relapse following CAR-T cell therapy and summarize potential strategies to overcome these obstacles, thus providing the possibility of improving standard treatment regimens.
Keywords: B-ALL; CAR-t; CD19; antigen escape; hematological malignancies; relapse.
Conflict of interest statement
Figures
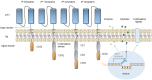
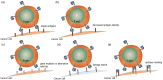
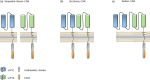
References
-
- Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18(8):712-725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

