A real-world experience of SARS-CoV-2 infection in a tertiary referral centre of Montréal: Unexpected low prevalence and low mortality
- PMID: 35989892
- PMCID: PMC9235123
- DOI: 10.3138/canlivj-2021-0022
A real-world experience of SARS-CoV-2 infection in a tertiary referral centre of Montréal: Unexpected low prevalence and low mortality
Abstract
Background: The impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with chronic liver disease (CLD) and liver transplant (LT) recipients remains a concern. The aim of this study was to report the impact of coronavirus disease 2019 (COVID-19) infection among patients at the tertiary health care centre Centre hospitalier de l'Université de Montréal (CHUM) during the first wave of the SARS-CoV-2 pandemic.
Methods: This real-world, retrospective cohort included all patients admitted to our liver unit and/or seen as an outpatient with CLD with or without cirrhosis and/or LT recipients who tested positive to SARS-CoV-2 infection. Cases were considered positive as defined by the detection of SARS-CoV-2 by reverse-transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swabs.
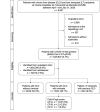
Results: Between April 1 and July 31, 2020, 5,637 were admitted to our liver unit and/or seen as outpatient. Among them, 42 were positive for SARS-CoV-2. Twenty-two patients had CLD without cirrhosis while 16 patients had cirrhosis at the time of the infection (13, 2, and 1 with Child-Pugh A, B, and C scores, respectively). Four were LT recipients. Overall, 15 of 42 patients (35.7%) were hospitalized; among them, 7 of 42 (16.7%) required respiratory support and 4 of 42 (9.5%) were transferred to the intensive care unit. Only 4 of 42 (9.5%) patients died: 2 with CLD without cirrhosis and 2 with CLD with cirrhosis. Overall survival was 90.5%.
Conclusion: This real-world study demonstrates an unexpectedly low prevalence and low mortality in the context of SARS-CoV-2 infection among patients with CLD with or without cirrhosis and LT recipients.
Keywords: COVID-19; SARS-CoV-2; chronic liver disease; cirrhosis; liver transplantation.
Copyright © 2021 Canadian Association for the Study of the Liver.
Conflict of interest statement
I Ruiz is a recipient of a fellowship grant from the Fondation du CHUM, Montréal, Québec, Canada. D Martel has served as an advisor and/or speaker for AbbVie and Gilead. The rest of the authors have no conflicts of interest to declare.
Figures

References
-
- Dumortier J, Duvoux C, Roux O, et al; French Solid Organ Transplant COVID Registry; Groupe de Recherche Français en Greffe de Foie (GReF). Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45(4):101639. Epub 2021 Jan 28. 10.1016/j.clinre.2021.101639. Medline: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
