A randomized single-blind controlled trial of a prototype digital polytherapeutic for tinnitus
- PMID: 35989940
- PMCID: PMC9389120
- DOI: 10.3389/fneur.2022.958730
A randomized single-blind controlled trial of a prototype digital polytherapeutic for tinnitus
Abstract
Objective: This randomized single-blind controlled trial tested the hypothesis that a prototype digital therapeutic developed to provide goal-based counseling with personalized passive and active game-based sound therapy would provide superior tinnitus outcomes, and similar usability, to a popular passive sound therapy app over a 12 week trial period.
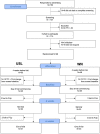
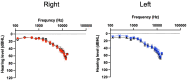
Methods: The digital therapeutic consisted of an app for iPhone or Android smartphone, Bluetooth bone conduction headphones, neck pillow speaker, and a cloud-based clinician dashboard to enable messaging and app personalization. The control app was a popular self-help passive sound therapy app called White Noise Lite (WN). The primary outcome measure was clinically meaningful change in Tinnitus Functional Index (TFI) between baseline and 12 weeks of therapy. Secondary tinnitus measures were the TFI total score and subscales across sessions, rating scales and the Client Oriented Scale of Improvement in Tinnitus (COSIT). Usability of the US and WN interventions were assessed using the System Usability Scale (SUS) and the mHealth App Usability Questionnaire (MAUQ). Ninety-eight participants who were smartphone app users and had chronic moderate-severe tinnitus (>6 months, TFI score > 40) were enrolled and were randomly allocated to one of the intervention groups. Thirty-one participants in the USL group and 30 in the WN group completed 12 weeks of trial.
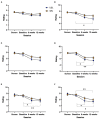
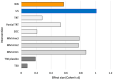
Results: Mean changes in TFI for the USL group at 6 (16.36, SD 17.96) and 12 weeks (17.83 points, SD 19.87) were clinically meaningful (>13 points reduction), the mean change in WN scores were not clinically meaningful (6 weeks 10.77, SD 18.53; 12 weeks 10.12 points, SD 21.36). A statistically higher proportion of USL participants achieved meaningful TFI change at 6 weeks (55%) and 12 weeks (65%) than the WN group at 6 weeks (33%) and 12 weeks (43%). Mean TFI, rating and COSIT scores favored the US group but were not statistically different from WN. Usability measures were similar for both groups.
Conclusions: The USL group demonstrated a higher proportion of responders than the WN group. The usability of the USL therapeutic was similar to the established WN app. The digital polytherapeutic demonstrated significant benefit for tinnitus reduction supporting further development.
Keywords: clinical trial; digital therapeutic; serious game; sound therapy; tinnitus.
Copyright © 2022 Searchfield and Sanders.
Conflict of interest statement
Author GS is a founder and scientific officer for TrueSilence a Spinout company of the University of Auckland and has a financial interest in TrueSilence. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Shahsavarani S, Khan RA, Husain FT. Tinnitus and the brain: a review of functional and anatomical magnetic resonance imaging studies. Perspect ASHA Spec Interest Groups. (2019) 4:896–909. 10.1044/2019_PERS-SIG6-2019-0001 - DOI
LinkOut - more resources
Full Text Sources

