Using intranasal dexmedetomidine with buccal midazolam for magnetic resonance imaging sedation in children: A single-arm prospective interventional study
- PMID: 35989987
- PMCID: PMC9386185
- DOI: 10.3389/fped.2022.889369
Using intranasal dexmedetomidine with buccal midazolam for magnetic resonance imaging sedation in children: A single-arm prospective interventional study
Abstract
Objective: Although numerous intravenous sedative regimens have been documented, the ideal non-parenteral sedation regimen for magnetic resonance imaging (MRI) has not been determined. This prospective, interventional study aimed to investigate the efficacy and safety of buccal midazolam in combination with intranasal dexmedetomidine in children undergoing MRI.
Methods: Children between 1 month and 10 years old requiring sedation for MRI examination were recruited to receive buccal midazolam 0.2 mg⋅kg-1 with intranasal dexmedetomidine 3 μg⋅kg-1. The primary outcome was successful sedation following the administration of the initial sedation regimens and the completion of the MRI examination.
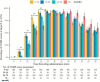
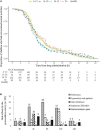
Results: Sedation with dexmedetomidine-midazolam was administered to 530 children. The successful sedation rate was 95.3% (95% confidence interval: 93.5-97.1%) with the initial sedation regimens and 97.7% (95% confidence interval: 96.5-99%) with a rescue dose of 2 μg⋅kg-1 intranasal dexmedetomidine. The median sedation onset time was 10 min, and a significant rising trend was observed in the onset time concerning age (R = 0.2491, P < 0.001). The wake-up and discharge times significantly correlated with the duration of the procedure (R = 0.323, P < 0.001 vs. R = 0.325, P < 0.001). No oxygen deficiency nor medication intervention due to cardiovascular instability was observed in any of the patients. History of a prior failed sedation was considered a statistically significant risk factor for failed sedation in the multivariate logistic regression model [odds ratio = 4.71 (95% confidence interval: 1.24-17.9), P = 0.023].
Conclusion: In MRI examinations, the addition of buccal midazolam to intranasal dexmedetomidine is associated with a high success rate and a good safety profile. This non-parenteral sedation regimen can be a feasible and convenient option for short-duration MRI in children between 1 month and 10 years.
Keywords: children; dexmedetomidine; magnetic resonance imaging; midazolam; sedation.
Copyright © 2022 Li, Luo, Huang, Zhang, Paquin, Yuen and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Combined sedation in pediatric magnetic resonance imaging: determination of median effective dose of intranasal dexmedetomidine combined with oral midazolam.BMC Anesthesiol. 2024 Mar 23;24(1):112. doi: 10.1186/s12871-024-02493-x. BMC Anesthesiol. 2024. PMID: 38521913 Free PMC article. Clinical Trial.
-
A randomized controlled trial of oral chloral hydrate vs intranasal dexmedetomidine plus buccal midazolam for auditory brainstem response testing in children.Paediatr Anaesth. 2018 Nov;28(11):1022-1028. doi: 10.1111/pan.13498. Epub 2018 Oct 3. Paediatr Anaesth. 2018. PMID: 30281180 Clinical Trial.
-
Comparison of oral triclofos and intranasal midazolam and dexmedetomidine for sedation in children undergoing magnetic resonance imaging (MRI): an open-label, three-arm, randomized trial.Eur J Pediatr. 2023 Mar;182(3):1385-1391. doi: 10.1007/s00431-022-04794-0. Epub 2023 Jan 20. Eur J Pediatr. 2023. PMID: 36658444 Free PMC article. Clinical Trial.
-
[Efficacy and safety of intranasal dexmedetomidine premedication for children undergoing CT or magnetic resonance imaging: a systematic review and meta-analysis].Zhonghua Er Ke Za Zhi. 2020 Apr 2;58(4):314-318. doi: 10.3760/cma.j.cn112140-20191224-00830. Zhonghua Er Ke Za Zhi. 2020. PMID: 32234139 Chinese.
-
Effects of dexmedetomidine sedation for magnetic resonance imaging in children: a systematic review and meta-analysis.J Anesth. 2021 Aug;35(4):525-535. doi: 10.1007/s00540-021-02946-4. Epub 2021 May 18. J Anesth. 2021. PMID: 34002258
Cited by
-
Combined use of intranasal Dexmedetomidine and an oral novel formulation of Midazolam for sedation of young children during brain MRI examination: a prospective, single-center, randomized controlled trial.BMC Anesthesiol. 2022 Nov 23;22(1):357. doi: 10.1186/s12871-022-01897-x. BMC Anesthesiol. 2022. PMID: 36418946 Free PMC article. Clinical Trial.
-
Revolutionizing Brain Drug Delivery: Buccal Transferosomes on the Verge of a Breakthrough.Recent Adv Drug Deliv Formul. 2024;18(4):262-275. doi: 10.2174/0126673878312336240802113811. Recent Adv Drug Deliv Formul. 2024. PMID: 39356098 Review.
-
The effect of age on outpatient pediatric procedural sedation with intranasal dexmedetomidine and oral midazolam.Eur J Pediatr. 2024 Jan;183(1):169-177. doi: 10.1007/s00431-023-05240-5. Epub 2023 Oct 19. Eur J Pediatr. 2024. PMID: 37855928 Free PMC article.
-
Intervention Bundle for Optimization of Procedural Sedation for Newborns Undergoing Magnetic Resonance Imaging: A Single-Center Quality Improvement Project in Qatar.Biomed Hub. 2024 May 22;9(1):73-82. doi: 10.1159/000538762. eCollection 2024 Jan-Dec. Biomed Hub. 2024. PMID: 39015198 Free PMC article.
-
Age-related characteristics of sedation in pediatric patients and their correlated adverse events: a cohort study.Front Pediatr. 2024 Dec 16;12:1475891. doi: 10.3389/fped.2024.1475891. eCollection 2024. Front Pediatr. 2024. PMID: 39741768 Free PMC article.
References
LinkOut - more resources
Full Text Sources

