Electrospun Nanofibers for Wound Management
- PMID: 35990019
- PMCID: PMC9384963
- DOI: 10.1002/cnma.202100349
Electrospun Nanofibers for Wound Management
Abstract
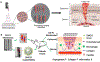
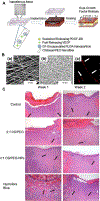
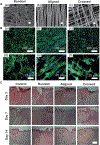
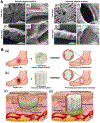
Electrospun nanofibers show great potential in biomedical applications. This mini review article traces the recent advances in electrospun nanofibers for wound management via various approaches. Initially, we provide a short note on the four phases of wound healing, including hemostasis, inflammation, proliferation, and remodeling. Then, we state how the nanofiber dressings can stop bleeding and reduce the pain. Following that, we discuss the delivery of therapeutics and cells using different types of nanofibers for enhancing cell migration, angiogenesis, and re-epithelialization, resulting in the promotion of wound healing. Finally, we present the conclusions and future perspectives regarding the use of electrospun nanofibers for wound management.
Keywords: Antibacterial; Electrospun nanofibers; Hemostasis; Pain relief; Wound healing.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures





References
-
- Broughton G, Janis JE, Attinger CE, Plast Reconstr. Surg 2006, 117, 12S–34S. - PubMed
-
- Ziegler TR, Pierce GF, Herndon DN, Growth Factors and Wound Healing New York: Springer, 2012, 1.
-
- Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K, Plast. Reconstr. Surg 2006, 117, 72S–109S. - PubMed
-
- Bandyk DF, Semin. Vasc. Surg 2018, 31, 43–48. - PubMed
-
- Ahmad J, Diabetes Metab. Syndr 2016, 10, 48–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
