First Evidence of the Double-Bond Formation by Deoxydehydration of Glycerol and 1,2-Propanediol in Ionic Liquids
- PMID: 35990467
- PMCID: PMC9386840
- DOI: 10.1021/acsomega.2c01803
First Evidence of the Double-Bond Formation by Deoxydehydration of Glycerol and 1,2-Propanediol in Ionic Liquids
Abstract
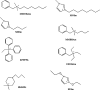
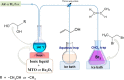
Deoxydehydration (DODH) reaction of glycerol (GL) and 1,2-propanediol (1,2-PD), in ionic liquids (ILs), catalyzed by methyltrioxorhenium (MTO) and Re2O7, was studied in detail. To better understand the ability of ILs to improve the catalytic performance of the rhenium catalyst, several experiments, employing eight different cations and two different anions, were carried out. Among the anions, bis(trifluoromethylsulfonyl)imide (TFSI) appears to be more appropriate than PF6 -, for its relatively lower volatility of the resulting IL. Regarding the choice of the most appropriate cation, the presence of a single aromatic ring seems to be a necessary requirement for a satisfying and convenient reactivity. With the aim to extend the recyclability of the catalyst, experiments involving the readdition of polyol to the terminal reaction mixture were carried out. Worthy of interest is the fact that the presence of the IL prevents the inertization process of the catalyst, allowing us to obtain the alkene also after a readdition of fresh polyol.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Garedew M.; Lin F.; Song B.; DeWinter T. M.; Jackson J. E.; Saffron C. M.; Lam C. H.; Anastas P. T. Greener routes to biomass waste valorization: Lignin transformation through electrocatalysis for renewable chemicals and fuels production. ChemSusChem 2020, 13, 4214–4237. 10.1002/cssc.202000987. - DOI - PubMed
-
- Dawes G. J. S.; Scott E. L.; Le Nôtre J.; Sanders J. P. M.; Bitter J. H. Deoxygenation of biobased molecules by decarboxylation and decarbonylation—a review on the role of heterogeneous, homogeneous and bio-catalysis. Green Chem. 2015, 17, 3231–3250. 10.1039/c5gc00023h. - DOI
-
- Salvi B. L.; Panwar N. L. Biodiesel resources and production technologies—A review. Renewable Sustainable Energy Rev. 2012, 16, 3680–3689. 10.1016/j.rser.2012.03.050. - DOI
-
- Possato L. G.; Chaves T. F.; Cassinelli W. H.; Pulcinelli S. H.; Santilli C. V.; Martins L. The multiple benefits of glycerol conversion to acrolein and acrylic acid catalyzed by vanadium oxides supported on micro-mesoporous MFI zeolites. Catal. Today 2017, 289, 20–28. 10.1016/j.cattod.2016.08.005. - DOI
-
- Mazarío J.; Concepción P.; Ventura M.; Domine M. E. Continuous catalytic process for the selective dehydration of glycerol over Cu-based mixed oxide. J. Catal. 2020, 385, 160–175. 10.1016/j.jcat.2020.03.010. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

