Synthesis and Evaluation of Pseudomonas aeruginosa ATP Synthase Inhibitors
- PMID: 35990476
- PMCID: PMC9386795
- DOI: 10.1021/acsomega.2c03127
Synthesis and Evaluation of Pseudomonas aeruginosa ATP Synthase Inhibitors
Abstract
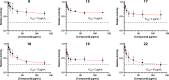
New antibiotics with unique biological targets are desperately needed to combat the growing number of resistant bacterial pathogens. ATP synthase, a critical protein found in all life, has recently become a target of interest for antibiotic development due to the success of the anti-tuberculosis drug bedaquiline, and while many groups have worked on developing drugs to target bacterial ATP synthase, few have been successful at inhibiting Pseudomonas aeruginosa (PA) ATP synthase specifically. PA is one of the leading causes of resistant nosocomial infections across the world and is extremely challenging to treat due to its various antibiotic resistance mechanisms for most commonly used antibiotics. Herein, we detail the synthesis and evaluation of a series of C1/C2 quinoline analogues for their ability to inhibit PA ATP synthase and act as antibiotics against wild-type PA. From this survey, we found six compounds capable of inhibiting PA ATP synthase in vitro showing that bulky/hydrophobic C1/C2 substitutions are preferred. The strongest inhibitor showed an IC50 of 10 μg/mL and decreased activity of PA ATP synthase to 24% relative to the control. While none of the compounds were able to inhibit wild-type PA in cell culture, two showed improved inhibition of PA growth when permeability of the outer membrane was increased or efflux was knocked out, thus demonstrating that these compounds could be further developed into efficacious antibiotics.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
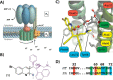



References
-
- CDC . Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention, 2019, p 148.
-
- MacGowan A.; Macnaughton E. Antibiotic Resistance. Medicine 2017, 45, 622–628. 10.1016/j.mpmed.2017.07.006. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

