PrEP uptake and HIV viral suppression when PrEP is integrated into Ugandan ART clinics for HIV-negative members of HIV-serodifferent couples: A stepped wedge cluster randomized trial
- PMID: 35990584
- PMCID: PMC9386395
- DOI: 10.1016/j.eclinm.2022.101611
PrEP uptake and HIV viral suppression when PrEP is integrated into Ugandan ART clinics for HIV-negative members of HIV-serodifferent couples: A stepped wedge cluster randomized trial
Abstract
Background: Global scale-up of HIV pre-exposure prophylaxis (PrEP) includes services to HIV-negative people in partnerships with people living with HIV (serodifferent couples). Data are needed on HIV outcomes, including uptake and adherence to PrEP and antiretroviral treatment (ART), to describe the impact of integrating PrEP into an existing HIV program.
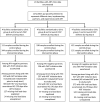
Methods: Using a stepped-wedge cluster randomized trial design, we launched PrEP delivery for HIV-negative members of serodifferent couples in Uganda by integrating PrEP into existing ART programs for people living with HIV. The program provided PrEP training for ART providers, ongoing technical assistance, and a provisional supply chain mechanism for PrEP medication. Primary data on PrEP initiation, PrEP refills, ART initiation, and HIV viremia at 6 months (measured at 42-270 days) were collected through data abstraction of medical records from HIV-serodifferent couples sequentially enrolling at the ART clinics. Modified Poisson regression models, controlling for time and cluster, compared viral suppression (<1000 copies/ml) before and after launch of the PrEP program. This trial was registered at ClinicalTrials.gov, NCT03586128.
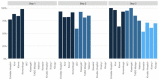
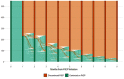
Findings: From June 1, 2018-December 15, 2020, 1,381 HIV-serodifferent couples were enrolled across 12 ART clinics in Kampala and Wakiso, Uganda, including 730 enrolled before and 651 after the launch of PrEP delivery. During the baseline period, 99.4% of partners living with HIV initiated ART and 85.0% were virally suppressed at 6 months. Among HIV-negative partners enrolled after PrEP launched, 81.0% (527/651) initiated PrEP within 90 days of enrolling; among these 527, 11.2% sought a refill 6 months later. In our powered intent-to-treat analysis, 82.1% and 76.7% of partners living with HIV were virally suppressed, respectively, which was not a statistically significant difference (RR=0.94, 95% CI: 0.82-1.07) and was stable across sensitivity analyses.
Interpretation: Integration of PrEP into ART clinics reached a high proportion of people in HIV-serodifferent relationships and did not improve the already high frequency of HIV viral suppression among partners living with HIV.
Funding: National Institute of Mental Health (R01MH110296).
Keywords: ART; PrEP; Serodifferent couples; Stepped-wedge trial; Viral suppression.
© 2022 The Authors.
Conflict of interest statement
JMB reports being an employee of Gilead Sciences, with salary and stock/options, outside the submitted work. All other authors declare no competing interests.
Figures
References
-
- Ministry of Health RoU . Ministry of Health; Kampala, Uganda: 2016. Technical Guidance on Pre-ExposureProphylaxis (PrEP) for Persons at High Risk of HIV in Uganda.
-
- World Health Organization . World Health Organization; Geneva, Switzerland: 2015. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

