Sensorimotor Integration and Pain Perception: Mechanisms Integrating Nociceptive Processing. A Systematic Review and ALE-Meta Analysis
- PMID: 35990591
- PMCID: PMC9390858
- DOI: 10.3389/fnint.2022.931292
Sensorimotor Integration and Pain Perception: Mechanisms Integrating Nociceptive Processing. A Systematic Review and ALE-Meta Analysis
Abstract
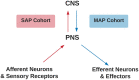
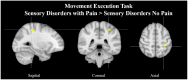
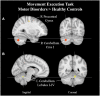
Pain treatment services and clinical indicators of pain chronicity focus on afferent nociceptive projections and psychological markers of pain perception with little focus on motor processes. Research supports a strong role for the motor system both in terms of pain related disability and in descending pain modulation. However, there is little understanding of the neurological regions implicated in pain-motor interactions and how the motor and sensory systems interact under conditions of pain. We performed an ALE meta-analysis on two clinical cohorts with atypical sensory and motor processes under conditions of pain and no pain. Persons with sensory altered processing (SAP) and no pain presented with greater activity in the precentral and supplementary motor area relative to persons with self-reported pain. In persons with motor altered processing (MAP), there appeared to be a suppression of activity in key pain regions such as the insula, thalamus, and postcentral gyrus. As such, activation within the motor system may play a critical role in dampening pain symptoms in persons with SAP, and in suppressing activity in key pain regions of the brain in persons with MAP. Future research endeavors should focus on understanding how sensory and motor processes interact both to understand disability and discover new treatment avenues.
Keywords: chronic pain; fMRI; motor system; nociceptive processing; pain perception; sensorimotor integration (SMI); sensorimotor processing and motor diseases.
Copyright © 2022 Gombaut and Holmes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

