Heat shock proteins and viral infection
- PMID: 35990630
- PMCID: PMC9389079
- DOI: 10.3389/fimmu.2022.947789
Heat shock proteins and viral infection
Abstract
Heat shock proteins (HSPs) are a kind of proteins which mostly found in bacterial, plant and animal cells, in which they are involved in the monitoring and regulation of cellular life activities. HSPs protect other proteins under environmental and cellular stress by regulating protein folding and supporting the correctly folded structure of proteins as chaperones. During viral infection, some HSPs can have an antiviral effect by inhibiting viral proliferation through interaction and activating immune pathways to protect the host cell. However, although the biological function of HSPs is to maintain the homeostasis of cells, some HSPs will also be hijacked by viruses to help their invasion, replication, and maturation, thereby increasing the chances of viral survival in unfavorable conditions inside the host cell. In this review, we summarize the roles of the heat shock protein family in various stages of viral infection and the potential uses of these proteins in antiviral therapy.
Keywords: HSPs; chaperones; immunological pathways; protein folding; viral infection.
Copyright © 2022 Zhang and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
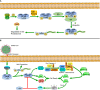
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

