Ferroptosis in sepsis: The mechanism, the role and the therapeutic potential
- PMID: 35990689
- PMCID: PMC9389368
- DOI: 10.3389/fimmu.2022.956361
Ferroptosis in sepsis: The mechanism, the role and the therapeutic potential
Abstract
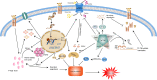
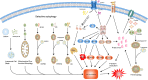
Sepsis is a common critical illness in the Intensive care unit(ICU) and its management and treatment has always been a major challenge in critical care medicine. The dysregulated host response to infection, causing systemic multi-organ and multi-system damage is the main pathogenesis. Notably, intense stress during sepsis can lead to metabolic disturbances of ions, lipids and energy in the organism. Ferroptosis is an iron-dependent, non-apoptotic cell death distinguished by a disruption of iron metabolism and iron-dependent accumulation of lipid peroxides. Mounting researches have established that ferroptosis has an essential part in anti-inflammatory and sepsis, and drugs targeting ferroptosis-related molecules, such as ferroptosis inhibitors, are gradually proving their effectiveness in sepsis. This paper summarizes and reviews the pathogenesis of ferroptosis, its regulatory network, and its vital involvement in the initiation of sepsis and related organ damage, and finally discusses the possible target drugs provided by the above mechanisms, describes the dilemmas as well as the outlook, in the hope of finding more links between ferroptosis and sepsis and providing new perspectives for the future treatment of sepsis.
Keywords: Nrf2; P53; autophagy; ferroptosis; mTOR; organ damage; sepsis; treatment.
Copyright © 2022 XL, GY, R and N.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

