Biocompatibility of titanium from the viewpoint of its surface
- PMID: 35990790
- PMCID: PMC9389932
- DOI: 10.1080/14686996.2022.2106156
Biocompatibility of titanium from the viewpoint of its surface
Abstract
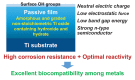
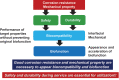
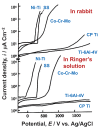
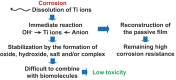
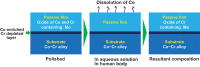
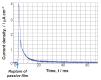
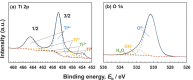
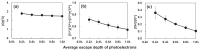
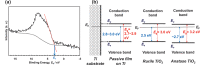
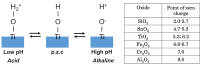
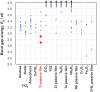
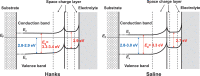
Among metals, Ti and majority of its alloys exhibit excellent biocompatibility or tissue compatibility. Although their high corrosion resistance is a factor in the biocompatibility of Ti and Ti alloys, it is clear that other factors exist. In this review, the corrosion resistance and passive film of Ti are compared to those of other metallic biomaterials, and their band gap energies, Egs, are compared to discuss the role of Eg in the reactivity with living tissues. From the perspective of the material's surface, it is possible to explain the excellent biocompatibility of Ti by considering the following factors: Ti ions are immediately stabilized not to show toxicity if it is released to body fluids; good balance of positive and negative charges by the dissociation of surface hydroxyl groups on the passive film; low electrostatic force of the passive film inducing a natural adsorption of proteins maintaining their natural conformation; strong property as n-type semiconductor; lower band gap energy of the passive film on Ti generating optimal reactivity; and calcium phosphate formation is caused by this reactivity. The results suggest that due to the passive oxide film, the optimal balance between high corrosion resistance and appropriate reactivity of Ti is the predominate solution for the excellent biocompatibility of Ti.
Keywords: Titanium; band gap energy; biocompatibility; corrosion resistance; electrostatic force; passive film; surface electric charge.
© 2022 The Author(s). Published by National Institute for Materials Science in partnership with Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Brunette DM, Tenvall P, Textor M, et al. Titanium in medicine. Berlin: Springer; 2001. DOI:10.1007/978-3-642-56486-4 - DOI
-
- Hanawa T. Zirconia versus titanium in dentistry: A review. Dent Mater J. 2020;39(1):24–36. - PubMed
-
- Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal—a review. J Prosth Dent. 2006;96(1):12. - PubMed
-
- William DF. Definitions in biomaterials. Proceedings of a Consensus Conference of the European Society for Biomaterials, Vol. 4, Chester, England, New York, NY: Elsevier; 1987.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
