The pleiotropic benefits of statins include the ability to reduce CD47 and amplify the effect of pro-efferocytic therapies in atherosclerosis
- PMID: 35990913
- PMCID: PMC9390974
- DOI: 10.1038/s44161-022-00023-x
The pleiotropic benefits of statins include the ability to reduce CD47 and amplify the effect of pro-efferocytic therapies in atherosclerosis
Abstract
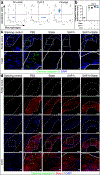
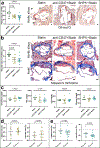
The pleiotropic benefits of statins may result from their impact on vascular inflammation. The molecular process underlying this phenomenon is not fully elucidated. Here, RNA sequencing designed to investigate gene expression patterns following CD47-SIRPα inhibition identifies a link between statins, efferocytosis, and vascular inflammation. In vivo and in vitro studies provide evidence that statins augment programmed cell removal by inhibiting the nuclear translocation of NFκB1 p50 and suppressing the expression of the critical 'don't eat me' molecule, CD47. Statins amplify the phagocytic capacity of macrophages, and thus the anti-atherosclerotic effects of CD47-SIRPα blockade, in an additive manner. Analyses of clinical biobank specimens suggest a similar link between statins and CD47 expression in humans, highlighting the potential translational implications. Taken together, our findings identify efferocytosis and CD47 as pivotal mediators of statin pleiotropy. In turn, statins amplify the anti-atherosclerotic effects of pro-phagocytic therapies independently of any lipid-lowering effect.
Keywords: Atherosclerosis; CD47; Efferocytosis; Pleiotropy; Statin.
Conflict of interest statement
Competing Interests I.L.W. and N.J.L. are co-founders and directors of Bitterroot Bio Incorporated, a cardiovascular company studying macrophage checkpoint inhibition. K.-U.J., Y.K., I.L.W., and N.J.L. have filed a provisional patent (U.S. Application Serial No. 63/106,794): ‘CD47 Blockade and Combination Therapies Thereof For Reduction Of Vascular Inflammation’. The remaining authors declare no competing interests.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
