Charge Maintenance during Catalysis in Nonheme Iron Oxygenases
- PMID: 35990992
- PMCID: PMC9387357
- DOI: 10.1021/acscatal.1c04770
Charge Maintenance during Catalysis in Nonheme Iron Oxygenases
Abstract
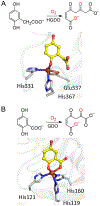
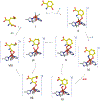
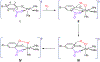
Here, the choice of the first coordination shell of the metal center is analyzed from the perspective of charge maintenance in a binary enzyme-substrate complex and an O2-bound ternary complex in the nonheme iron oxygenases. Comparing homogentisate 1,2-dioxygenase and gentisate dioxygenase highlights the significance of charge maintenance after substrate binding as an important factor that drives the reaction coordinate. We then extend the charge analysis to several common types of nonheme iron oxygenases containing either a 2-His-1-carboxylate facial triad or a 3-His or 4-His ligand motif, including extradiol and intradiol ring-cleavage dioxygenases, thiol dioxygenases, α-ketoglutarate-dependent oxygenases, and carotenoid cleavage oxygenases. After forming the productive enzyme-substrate complex, the overall charge of the iron complex at the 0, +1, or +2 state is maintained in the remaining catalytic steps. Hence, maintaining a constant charge is crucial to promote the reaction of the iron center beginning from the formation of the Michaelis or ternary complex. The charge compensation to the iron ion is tuned not only by protein-derived carboxylate ligands but also by substrates. Overall, these analyses indicate that charge maintenance at the iron center is significant when all the necessary components form a productive complex. This charge maintenance concept may apply to most oxygen-activating metalloenzymes systems that do not draw electrons and protons step-by-step from a separate reactant, such as NADH, via a reductase. The charge maintenance perception may also be useful in proposing catalytic pathways or designing prototypical reactions using artificial or engineered enzymes for biotechnological applications.
Keywords: biocatalysis; charge compensation; electron transfer; iron catalysts; oxidation; oxygenation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
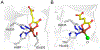

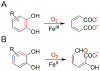





References
-
- Solomon EI; Brunold TC; Davis MI; Kemsley JN; Lee SK; Lehnert N; Neese F; Skulan AJ; Yang YS; Zhou J Geometric and Electronic Structure/Function Correlations in Nonheme Iron Enzymes. Chem. Rev 2000, 100, 235–350. - PubMed
-
- Furukawa K Engineering Dioxygenases for Efficient Degradation of Environmental Pollutants. Curr. Opin. Biotechnol 2000, 11, 244–249. - PubMed
-
- Costas M; Mehn MP; Jensen MP; Que L Dioxygen Activation at Mononuclear Non-heme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev 2004, 104, 939–986. - PubMed
-
- Vaillancourt FH; Bolin JT; Eltis LD The Ins and Outs of Ring-cleaving Dioxygenases. Crit. Rev. Biochem. Mol. Biol 2006, 41, 241–267. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
