Pooled surveillance testing for asymptomatic SARS-CoV-2 infections at a Veterinary Teaching Hospital College, University of Minnesota, December 2020-April 2021
- PMID: 35991058
- PMCID: PMC9388852
- DOI: 10.3389/fpubh.2022.879107
Pooled surveillance testing for asymptomatic SARS-CoV-2 infections at a Veterinary Teaching Hospital College, University of Minnesota, December 2020-April 2021
Abstract
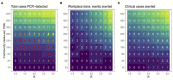
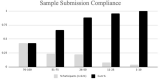
To evaluate the use of asymptomatic surveillance, we implemented a surveillance program for asymptomatic SARS-CoV-2 infection in a voluntary sample of individuals at the College of Veterinary Medicine at the University of Minnesota. Self-collected anterior nasal samples were tested using real time reverse transcription-polymerase chain reaction (RT-PCR), in a 5:1 pooled testing strategy, twice weekly for 18 weeks. Positive pools were deconvoluted into individual tests, revealing an observed prevalence of 0.07% (3/4,525). Pooled testing allowed for large scale testing with an estimated cost savings of 79.3% and modeling demonstrated this testing strategy prevented up to 2 workplace transmission events, averting up to 4 clinical cases. At the study endpoint, antibody testing revealed 80.7% of participants had detectable vaccine antibody levels while 9.6% of participants had detectable antibodies to natural infection.
Keywords: COVID-19; SARS-CoV-2; cost effective; pooled testing; serology; surveillance; veterinary medicine.
Copyright © 2022 Mladonicky, Bedada, Yoder, VanderWaal, Torrison and Wells.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- CDC . COVID-19 Pandemic Planning Scenarios. Centers for Disease Control and Prevention. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html (accessed August 30, 2021).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

