The F1Fo-ATPase inhibitor protein IF1 in pathophysiology
- PMID: 35991181
- PMCID: PMC9389554
- DOI: 10.3389/fphys.2022.917203
The F1Fo-ATPase inhibitor protein IF1 in pathophysiology
Abstract
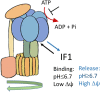
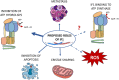
The endogenous inhibitor of ATP synthase is a protein of about 10 kDa, known as IF1 which binds to the catalytic domain of the enzyme during ATP hydrolysis. The main role of IF1 consists of limiting ATP dissipation under condition of severe oxygen deprivation or in the presence of dysfunctions of mitochondrial respiratory complexes, causing a collapse in mitochondrial membrane potential and therefore ATP hydrolysis. New roles of IF1 are emerging in the fields of cancer and neurodegeneration. Its high expression levels in tumor tissues have been associated with different roles favouring tumor formation, progression and evasion. Since discordant mechanisms of action have been proposed for IF1 in tumors, it is of the utmost importance to clarify them in the prospective of defining novel approaches for cancer therapy. Other IF1 functions, including its involvement in mitophagy, may be protective for neurodegenerative and aging-related diseases. In the present review we aim to clarify and discuss the emerging mechanisms in which IF1 is involved, providing a critical view of the discordant findings in the literature.
Keywords: ATP synthase; cancer; inhibitor protein IF1; mitochondria; neurodegeneration.
Copyright © 2022 Gatto, Grandi, Solaini, Baracca and Giorgio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Atwal K. S., Wang P., Rogers W. L., Sleph P., Monshizadegan H., Ferrara F. N., et al. (2004). Small molecule mitochondrial F 1F 0 ATPase hydrolase inhibitors as cardioprotective agents. Identification of 4-(n-Arylimidazole)-Substituted benzopyran derivatives as selective hydrolase inhibitors. J. Med. Chem. 47, 1081–1084. 10.1021/jm030291x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

