Comparison of Ondansetron versus Domperidone for treating vomiting in acute gastroenteritis in children at a resource limited setting of South Punjab, Pakistan
- PMID: 35991241
- PMCID: PMC9378405
- DOI: 10.12669/pjms.38.6.5532
Comparison of Ondansetron versus Domperidone for treating vomiting in acute gastroenteritis in children at a resource limited setting of South Punjab, Pakistan
Abstract
Objectives: To compare the efficacy of Ondansetron versus Domperidone for treating vomiting in acute gastroenteritis (AGE) in children at a resource limited emergency setting of South Punjab, Pakistan.
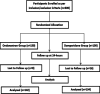
Methods: This open label randomized controlled trial was conducted at The Pediatric Emergency Department of Tehshil Headquarter Hospital, Liaqatpur, Pakistan, from July 2020 to June 2021. A total of 300 children of both genders aged below 12 years of age having 3 or more non-bilious, non-bloody vomiting episodes within 24 hours and with suggestive signs and symptoms of AGE were enrolled and randomized (150 in each group). Efficacy of both drugs was compared in terms of need of 2nd dose within 15 minutes, cessation of vomiting at 6-hour and 24-hour follow up.
Results: Out of a total of 300 children, 162 (54.0%) were male. Mean age was 4.7±2.3 years. Twenty seven (18.0%) children in Ondansetron group required 2nd dose within 15 minutes while 38 (25.3%) children in Domperidone group required the 2nd dose (p=0.1232). Cessation of vomiting at 6-hour interval was noted among 126 (84.0%) children in Ondansetron group in comparison to 118 (78.7%) in Domperidone group (p=0.2359). It was revealed that 127/142 (89.4%) children in Ondansetron group had cessation of vomiting at 24-hours follow up while this was noted to be among 108/134 (80.6%) children in Domperidone group (p=0.0390).
Conclusion: In comparison to Domperidone, Ondansetron was found to have better efficacy aiming cessation of AGE associated vomiting among children with mild to moderate dehydration.
Keywords: Acute gastroenteritis; Domperidone; Ondansetron; dehydration.
Copyright: © Pakistan Journal of Medical Sciences.
Conflict of interest statement
Conflict of Interest: None.
References
-
- Seo JH, Shim JO, Choe BH, Moon JS, Kang KS, Chung JY. Management of acute gastroenteritis in children:A survey among members of the Korean Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Pediatr Gastroenterol Hepatol Nutr. 2019;22(5):431–440. doi:10.5223/pghn.2019.22.5.431. - PMC - PubMed
-
- Malek MA, Curns AT, Holman RC, Fischer TK, Bresee JS, Glass RI, et al. Diarrhea and rotavirus-associated hospitalizations among children less than 5 years of age:United States,1997 2000. Pediatrics. 2006;117:1887–1892. doi:10.1542/peds.2005-2351. - PubMed
-
- Acharya D, Singh JK, Adhikari M, Gautam S, Pandey P, Dayal V. Association of water handling and child feeding practice with childhood diarrhoea in rural community of Southern Nepal. J Infect Public Health. 2018;11:69–74. doi:10.1016/j.jiph.2017.04.007. - PubMed
LinkOut - more resources
Full Text Sources