3D Printability Assessment of Poly(octamethylene maleate (anhydride) citrate) and Poly(ethylene glycol) Diacrylate Copolymers for Biomedical Applications
- PMID: 35991303
- PMCID: PMC9379906
- DOI: 10.1021/acsapm.2c00531
3D Printability Assessment of Poly(octamethylene maleate (anhydride) citrate) and Poly(ethylene glycol) Diacrylate Copolymers for Biomedical Applications
Abstract
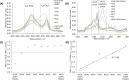
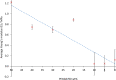
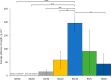
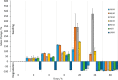
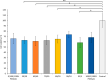
Herein, we present the first example of 3D printing with poly(octamethylene maleate (anhydride) citrate) (POMaC), a bio-adhesive material which has shown particular promise for implantable biomedical devices. The current methods to fabricate such devices made from POMaC are hindered by the imposed constraints of designing complex molds. We demonstrate the feasibility of exploiting additive manufacturing to 3D print structural functional materials consisting of POMaC. We present 3D printing of biomaterial copolymers consisting of mixtures of poly(ethylene glycol) diacrylate (PEGDA) and POMaC at different ratios. The required parameters were optimized, and characterization of the printing fidelity and physical properties was performed. We have also demonstrated that a range of mechanical properties can be achieved by tuning the POMaC/PEGDA ratio. The biocompatibility of the copolymers was ascertained via a cell viability assay. Such tunable 3D printed biomaterials consisting of POMaC and PEGDA will have significant potential application in the development of functional biomaterial tissue scaffolds and biomedical devices for the future of personalized medicine.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
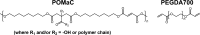



Similar articles
-
Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism.Soft Matter. 2010 Jan 1;6(11):2449-2461. doi: 10.1039/C001605E. Soft Matter. 2010. PMID: 22162975 Free PMC article.
-
High-Resolution Additive Manufacturing of a Biodegradable Elastomer with A Low-Cost LCD 3D Printer.Adv Healthc Mater. 2024 Apr;13(9):e2303708. doi: 10.1002/adhm.202303708. Epub 2023 Dec 7. Adv Healthc Mater. 2024. PMID: 37990819 Free PMC article.
-
Control of maleic acid-propylene diepoxide hydrogel for 3D printing application for flexible tissue engineering scaffold with high resolution by end capping and graft polymerization.Biomater Res. 2022 Dec 9;26(1):75. doi: 10.1186/s40824-022-00318-x. Biomater Res. 2022. PMID: 36494708 Free PMC article.
-
Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering.Polymers (Basel). 2023 May 17;15(10):2341. doi: 10.3390/polym15102341. Polymers (Basel). 2023. PMID: 37242919 Free PMC article. Review.
-
The Current Versatility of Polyurethane Three-Dimensional Printing for Biomedical Applications.Tissue Eng Part B Rev. 2020 Jun;26(3):272-283. doi: 10.1089/ten.TEB.2019.0224. Tissue Eng Part B Rev. 2020. PMID: 32089089 Review.
Cited by
-
Porous Hydrogels for Immunomodulatory Applications.Int J Mol Sci. 2024 May 9;25(10):5152. doi: 10.3390/ijms25105152. Int J Mol Sci. 2024. PMID: 38791191 Free PMC article. Review.
-
Fabrication and Characterization of Brain Tissue Phantoms Using Agarose Gels for Ultraviolet Vision Systems.Gels. 2024 Aug 20;10(8):540. doi: 10.3390/gels10080540. Gels. 2024. PMID: 39195069 Free PMC article.
-
Rheological Properties and 3D Printing Behavior of PCL and DMSO2 Composites for Bio-Scaffold.Materials (Basel). 2024 May 20;17(10):2459. doi: 10.3390/ma17102459. Materials (Basel). 2024. PMID: 38793525 Free PMC article.
-
Hydrogel Microarray for Bioanalytical Applications: Preliminary Study on Material Properties.Materials (Basel). 2025 Jul 1;18(13):3118. doi: 10.3390/ma18133118. Materials (Basel). 2025. PMID: 40649606 Free PMC article.
-
Anisotropic Photoelectric Properties of Aligned P3HT Nanowire Arrays Fabricated via Solution Blade Coating and UV-Induced Molecular Ordering.Materials (Basel). 2025 Jun 5;18(11):2649. doi: 10.3390/ma18112649. Materials (Basel). 2025. PMID: 40508646 Free PMC article.
References
-
- Yang J.; Webb A. R.; Ameer G. A. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv. Mater. 2004, 16, 511–516. 10.1002/adma.200306264. - DOI
-
- Tran R.; Zhang Y.; Gyawali D.; Yang J. Recent Developments on Citric Acid Derived Biodegradable Elastomers. Recent Pat. Biomed. Eng. 2009, 2, 216–227. November 110.2174/1874764710902030216. - DOI
LinkOut - more resources
Full Text Sources

