The complex role of kininogens in hereditary angioedema
- PMID: 35991308
- PMCID: PMC9382879
- DOI: 10.3389/falgy.2022.952753
The complex role of kininogens in hereditary angioedema
Abstract
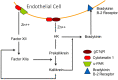
Human high molecular weight kininogen (HK) is the substrate from which bradykinin is released as a result of activation of the plasma "contact" system, a cascade that includes the intrinsic coagulation pathway, and a fibrinolytic pathway leading to the conversion of plasminogen to plasmin. Its distinction from low molecular weight kininogen (LK) was first made clear in studies of bovine plasma. While early studies did suggest two kininogens in human plasma also, their distinction became clear when plasma deficient in HK or both HK and LK were discovered. The light chain of HK is distinct and has the site of interaction with negatively charged surfaces (domain 5) plus a 6th domain that binds either prekallikrein or factor XI. HK is a cofactor for multiple enzymatic reactions that relate to the light chain binding properties. It augments the rate of conversion of prekallikrein to kallikrein and is essential for the activation of factor XI. It indirectly augments the "feedback" activation of factor XII by plasma kallikrein. Thus, HK deficiency has abnormalities of intrinsic coagulation and fibrinolysis akin to that of factor XII deficiency in addition to the inability to produce bradykinin by factor XII-dependent reactions. The contact cascade binds to vascular endothelial cells and HK is a critical binding factor with binding sites within domains 3 and 5. Prekallikrein (or factor XI) is attached to HK and is brought to the surface. The endothelial cell also secretes proteins that interact with the HK-prekallikrein complex resulting in kallikrein formation. These have been identified to be heat shock protein 90 (HSP 90) and prolylcarboxypeptidase. Cell release of urokinase plasminogen activator stimulates fibrinolysis. There are now 6 types of HAE with normal C1 inhibitors. One of them has a mutated kininogen but the mechanism for overproduction (presumed) of bradykinin has not yet been determined. A second has a mutation involving sulfation of proteoglycans which may lead to augmented bradykinin formation employing the cell surface reactions noted above.
Keywords: angioedema; bradykinin; kallikrein; kininogen; vascular permeability.
Copyright © 2022 Kaplan, Joseph and Ghebrehiwet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Komiya M, Kato H, Suzuki T. Bovine plasma kininogens. III Structural comparison of high molecular weight and low molecular weight kininogens. J Biochem. (1974) 76:833–45. - PubMed
-
- Spragg J, Austen K. The preparation of human kininogen. II Further characterization of purified human kininogen. J Immunol. (1971) 107:1512–9. - PubMed
-
- Pierce J. Structural features of plasma kinins and kininogens. Fed Proc. (1968) 27:52–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

