Immunomodulatory effect and safety of TNF-α RNAi mediated by oral yeast microcapsules in rheumatoid arthritis therapy
- PMID: 35991628
- PMCID: PMC9386491
- DOI: 10.1016/j.mtbio.2022.100384
Immunomodulatory effect and safety of TNF-α RNAi mediated by oral yeast microcapsules in rheumatoid arthritis therapy
Abstract
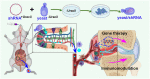
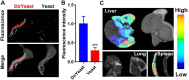
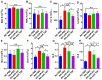
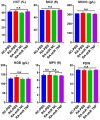
Rheumatoid arthritis (RA) is a chronic autoimmune disease that requires long-term treatment and monitoring. Inhibition of inflammatory gene expression by gene therapy is a significant breakthrough in RA treatment, but the lack of a safe and effective gene delivery system hinders its application. Since oral administration can significantly reduce wound infection caused by parenteral administration, it also has the advantages of high patient compliance and convenience. Therefore, oral administration may be the best option for the treatment of this chronic disease. In this study, we developed a novel oral drug system by delivering tumor necrosis factor-α (TNF-α) short hairpin RNA (shRNA) mediated by non-pathogenic yeast to evaluate its regulation of systemic immune inflammation and safety in RA. Non-pathogenic yeast can resist the destruction of the gastrointestinal acid-base environment and can be recognized by the intestinal macrophages and act on systemic inflammatory lesions. Oral administration of yeast-mediated TNF-α shRNA significantly reduced the expression of TNF-α predominant pro-inflammatory factors in intestinal macrophages and joint synovium, and up-regulated the expression of anti-inflammatory cytokine IL-10 and M2 macrophages, systematically regulating the inflammatory response. This yeast-mediated oral gene delivery system can not only significantly inhibit knee joint synovial inflammation, but also has no toxic effects on peripheral blood and major organs. Therefore, yeast-mediated oral delivery of TNF-α shRNA may be used as a novel gene therapy strategy to treat RA through immunomodulating the mononuclear phagocyte system from the intestine to the joint synovium, and ultimately regulating systemic and local immune inflammation, providing new ideas for the clinical treatment of RA.
Keywords: Gene therapy; Immunomodulation; Oral administration; Rheumatoid arthritis.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Yeast microcapsule-mediated oral delivery of IL-1β shRNA for post-traumatic osteoarthritis therapy.Mol Ther Nucleic Acids. 2020 Nov 11;23:336-346. doi: 10.1016/j.omtn.2020.11.006. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33425491 Free PMC article.
-
Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-12. doi: 10.11124/jbisrir-2012-288. JBI Libr Syst Rev. 2012. PMID: 27820156
-
Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned?Annu Rev Immunol. 2001;19:163-96. doi: 10.1146/annurev.immunol.19.1.163. Annu Rev Immunol. 2001. PMID: 11244034 Review.
-
Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis.Bone. 2002 Feb;30(2):340-6. doi: 10.1016/s8756-3282(01)00682-2. Bone. 2002. PMID: 11856640 Review.
Cited by
-
Research Advances in Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy.Pharmaceutics. 2023 Apr 13;15(4):1237. doi: 10.3390/pharmaceutics15041237. Pharmaceutics. 2023. PMID: 37111722 Free PMC article. Review.
-
Understanding yeast shells: structure, properties and applications.ADMET DMPK. 2024 Feb 15;12(2):299-317. doi: 10.5599/admet.2118. eCollection 2024. ADMET DMPK. 2024. PMID: 38720922 Free PMC article. Review.
-
Plasma levels of miR-21b and miR-146a can discriminate rheumatoid arthritis diagnosis and severity.Biomedicine (Taipei). 2025 Mar 1;15(1):30-41. doi: 10.37796/2211-8039.1637. eCollection 2025. Biomedicine (Taipei). 2025. PMID: 40176861 Free PMC article.
-
New Dawn in the Treatment of Rheumatoid Arthritis: Advanced Insight into Polymer Hydrogel Research.Gels. 2025 Feb 15;11(2):136. doi: 10.3390/gels11020136. Gels. 2025. PMID: 39996679 Free PMC article. Review.
References
-
- Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. - PubMed
-
- Xiang Y.J., Dai S.M. Prevalence of rheumatic diseases and disability in China. Rheumatol. Int. 2009;29(5):481–490. - PubMed
-
- Li R., Sun J., Ren L.M., Wang H.Y., Liu W.H., Zhang X.W., Chen S., Mu R., He J., Zhao Y., Long L., Liu Y.Y., Liu X., Lu X.L., Li Y.H., Wang S.Y., Pan S.S., Li C., Wang H.Y., Li Z.G. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology. 2012;51(4):721–729. - PubMed
-
- Lacaille D., Sheps S., Spinelli J.J., Chalmers A., Esdaile J.M. Identification of modifiable work-related factors that influence the risk of work disability in rheumatoid arthritis. Arthritis Rheum. 2004;51(5):843–852. - PubMed
-
- Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344(12):907–916. - PubMed
LinkOut - more resources
Full Text Sources

