Lidocaine and Ketamine Infusions as Adjunctive Pain Management Therapy: A Retrospective Analysis of Clinical Outcomes in Hospitalized Patients Admitted for Pain Related to Sickle Cell Disease
- PMID: 35992021
- PMCID: PMC9386131
- DOI: 10.3389/fpain.2022.878985
Lidocaine and Ketamine Infusions as Adjunctive Pain Management Therapy: A Retrospective Analysis of Clinical Outcomes in Hospitalized Patients Admitted for Pain Related to Sickle Cell Disease
Abstract
Objective: In this study, we aim to evaluate the efficacy of adjunctive lidocaine and ketamine infusions for opioid reduction in the treatment of sickle cell disease in patients with vaso-occlusive crisis (VOC).
Design: We retrospectively reviewed a cohort of 330 adult sickle-cell crisis hospital encounters with 68 patients admitted to our institution from July 2017 to August 2018.
Methods: Upon institutional IRB approval, we obtained initial data from billing records and performed chart reviews to obtain pain scores and confirm total opioid consumption. If provided by the acute pain consultation service, the patients received either a lidocaine or a ketamine infusion of 0.5-2 mg/min or 2-3 mcg/kg, respectively, for a maximum of 24-48 h. We compared the change in opioid consumption before and after infusion therapy to patients that did not receive ketamine or lidocaine.
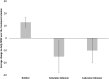
Results: Compared to patients that did not receive infusion therapy, ketamine and lidocaine accounted for respective relative decreases of 28 and 23% in average daily morphine consumption (p = 0.02). Patients that received either infusion were 3 to 4 times more likely to decrease their opioid consumption independent of treatment length or baseline opioid doses (p < 0.01). Ketamine and lidocaine therapies were not associated with change in pain scores. When a patient had multiple admissions, opioid reduction was strongly correlated with initiation of infusions in the later visits.
Conclusion: Both ketamine and lidocaine infusion therapies are effective in reducing opioid consumption for patients with vaso-occlusive crisis. Lidocaine infusion is emerging as an agent for stabilizing opioid doses in VOC for patients with high daily MME.
Keywords: lidocaine infusion; opioid dose reduction; opioid tolerance; sickle cell disease; vaso-occlusive crisis.
Copyright © 2022 Zavala, Knoebel and Anitescu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease.Curr Opin Support Palliat Care. 2019 Dec;13(4):402-407. doi: 10.1097/SPC.0000000000000437. Curr Opin Support Palliat Care. 2019. PMID: 31157658
-
Early Initiation of Sub-Anesthetic Ketamine Infusion in Adults with Vaso-Occlusive Crises Is Associated with Greater Reduction in Sickle Cell Pain Intensity: A Single Center's Experience.Pain Med. 2022 Dec 1;23(12):2042-2049. doi: 10.1093/pm/pnac094. Pain Med. 2022. PMID: 35708641 Free PMC article.
-
Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: a randomized controlled trial.Scand J Pain. 2018 Jan 26;18(1):19-27. doi: 10.1515/sjpain-2017-0140. Scand J Pain. 2018. PMID: 29794277 Clinical Trial.
-
Low-dose ketamine as a potential adjuvant therapy for painful vaso-occlusive crises in sickle cell disease.Paediatr Anaesth. 2013 Aug;23(8):684-9. doi: 10.1111/pan.12172. Epub 2013 Apr 9. Paediatr Anaesth. 2013. PMID: 23565738 Review.
-
Ketamine for Sickle Cell Vaso-Occlusive Crises: A Systematic Review.Saudi J Med Med Sci. 2021 Jan-Apr;9(1):3-9. doi: 10.4103/sjmms.sjmms_218_20. Epub 2020 Dec 26. Saudi J Med Med Sci. 2021. PMID: 33519337 Free PMC article. Review.
Cited by
-
Beyond IV push: alternative methods for management of acute pain in SCD.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):611-617. doi: 10.1182/hematology.2024000585. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39644010 Free PMC article. Review.
-
Emergency department pain management in special populations.Turk J Emerg Med. 2025 Jul 1;25(3):159-177. doi: 10.4103/tjem.tjem_141_25. eCollection 2025 Jul-Sep. Turk J Emerg Med. 2025. PMID: 40746577 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

