Practical and thermodynamic constraints on electromicrobially accelerated CO2 mineralization
- PMID: 35992063
- PMCID: PMC9385556
- DOI: 10.1016/j.isci.2022.104769
Practical and thermodynamic constraints on electromicrobially accelerated CO2 mineralization
Abstract
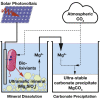
By the end of the century, tens of gigatonnes of CO2 will need to be removed from the atmosphere every year to maintain global temperatures. Natural weathering of ultramafic rocks and subsequent mineralization reactions can convert CO2 into ultra-stable carbonates. Although this will draw down all excess CO2, it will take thousands of years. CO2 mineralization could be accelerated by weathering ultramafic rocks with biodegradable lixiviants. We show that if these lixiviants come from cellulosic biomass, this demand could monopolize the world's biomass supply. We demonstrate that electromicrobial production technologies (EMP) that combine renewable electricity and microbial metabolism could produce lixiviants for as little as $200 to $400 per tonne at solar electricity prices achievable within the decade. We demonstrate that EMP could make enough lixiviants to sequester a tonne of CO2 for less than $100. This work highlights the potential of this approach and the need for extensive R&D.
Keywords: Biotechnology; Energy sustainability; Engineering; Microbiology.
© 2022 The Author(s).
Conflict of interest statement
B.B. is a contributor to REEgen, Inc., which is developing genetically engineered microbes for mineral-dissolution for rare earth element bio-mining, and will be a member of its scientific advisory board (it has yet to be formed as of the time of writing). B.B. is pursuing patent protection for engineered organisms for rare earth bio-mining (US provisional application 63/220,475). Although the specifics of ultramafic rock dissolution are likely to be different from the dissolution of rare earth-containing minerals, dissolving ultramafic rock is a logical extension of this technology. We believe this constitutes a sufficient perception of conflict of interest to warrant mention.
Figures




References
-
- Appel A.M., Bercaw J.E., Bocarsly A.B., Dobbek H., Dubois D.L., Dupuis M., Ferry J.G., Fujita E., Hille R., Kenis P.J.A., et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 2013;113:6621–6658. doi: 10.1021/cr300463y. - DOI - PMC - PubMed
-
- Archer D., Eby M., Brovkin V., Ridgwell A., Cao L., Mikolajewicz U., Caldeira K., Matsumoto K., Munhoven G., Montenegro A., Tokos K. Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet Sci. 2009;37:117–134. doi: 10.1146/annurev.earth.031208.100206. - DOI
-
- Barstow B. Molecular mechanisms for the biological storage of renewable energy. Adv. Sci. Eng. Med. 2015;7:1066–1081. doi: 10.1166/asem.2015.1813. - DOI
-
- Barstow B. 2021. ElectroCO2 Release for Zenodo. - DOI
LinkOut - more resources
Full Text Sources

