Chemoproteomics reveals berberine directly binds to PKM2 to inhibit the progression of colorectal cancer
- PMID: 35992091
- PMCID: PMC9386086
- DOI: 10.1016/j.isci.2022.104773
Chemoproteomics reveals berberine directly binds to PKM2 to inhibit the progression of colorectal cancer
Abstract
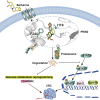
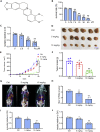
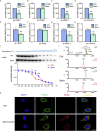
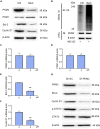
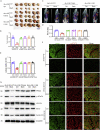
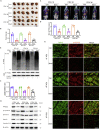
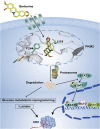
Colorectal cancer is one of the most serious tumors and berberine can inhibit the recurrence and transformation of colorectal adenoma into colorectal cancer. However, the direct binding target proteins of berberine in inhibiting colorectal cancer remain unclear. In this study, the chemical proteomics method was used and demonstrated that berberine is directly bound to pyruvate kinase isozyme type M2 (PKM2) in colorectal cancer cells. The triangular N-O-O triangular structure of berberine contributed to hydrophobic interaction with I119 amino acid residues and π-π interaction with F244 amino acid residues of PKM2 protein. Moreover, berberine was shown to inhibit the reprogramming of glucose metabolism and the phosphorylation of STAT3, down regulate the expression of Bcl-2 and Cyclin D1 genes, ultimately inhibiting the progression of colorectal cancer. This study uncovered the direct binding target protein and mechanism of berberine to improve metabolic reprogramming in colorectal cancer, which is helpful to guide the optimization of berberine.
Keywords: Biochemistry; Biomolecules; Molecular biology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abegg D., Frei R., Cerato L., Prasad Hari D., Wang C., Waser J., Adibekian A. Proteome-Wide profiling of targets of cysteine reactive small molecules by using ethynyl benziodoxolone reagents. Angew. Chem. Int. Ed. Engl. 2015;54:10852–10857. - PubMed
-
- Bessi I., Bazzicalupi C., Richter C., Jonker H.R.A., Saxena K., Sissi C., Chioccioli M., Bianco S., Bilia A.R., Schwalbe H., Gratteri P. Spectroscopic, molecular modeling, and NMR-spectroscopic investigation of the binding mode of the natural alkaloids berberine and sanguinarine to human telomeric G-quadruplex DNA. ACS Chem. Biol. 2012;7:1109–1119. - PubMed
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer. J. Clin. 2018;68:394–424. - PubMed
-
- Case D.A., Betz R.M., Cerutti D.S., Cheatham T.E., Darden T.A., III, Duke R.E., Giese T.J., Gohlke H., Goetz A.W., Homeyer N., et al. University of California; 2016. AMBER 2016.
-
- Chen C., Tao C., Liu Z., Lu M., Pan Q., Zheng L., Li Q., Song Z., Fichna J. A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrom. Phytother Res. 2015;29:1822–1827. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

