Female fertility does not require Bmal1 in suprachiasmatic nucleus neurons expressing arginine vasopressin, vasoactive intestinal peptide, or neuromedin-S
- PMID: 35992114
- PMCID: PMC9389073
- DOI: 10.3389/fendo.2022.956169
Female fertility does not require Bmal1 in suprachiasmatic nucleus neurons expressing arginine vasopressin, vasoactive intestinal peptide, or neuromedin-S
Abstract
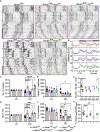
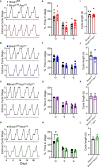
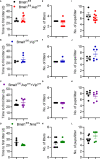
Disruptions to the circadian system alter reproductive capacity, particularly in females. Mice lacking the core circadian clock gene, Bmal1, are infertile and have evidence of neuroendocrine disruption including the absence of the preovulatory luteinizing hormone (LH) surge and enhanced responsiveness to exogenous kisspeptin. Here, we explore the role of Bmal1 in suprachiasmatic nucleus (SCN) neuron populations known to project to the neuroendocrine axis. We generated four mouse lines using Cre/Lox technology to create conditional deletion of Bmal1 in arginine vasopressin (Bmal1fl/fl:Avpcre ), vasoactive intestinal peptide (Bmal1fl/fl:Vipcre ), both (Bmal1fl/fl:Avpcre+Vipcre ), and neuromedin-s (Bmal1fl/fl:Nmscre ) neurons. We demonstrate that the loss of Bmal1 in these populations has substantial effects on home-cage circadian activity and temperature rhythms. Despite this, we found that female mice from these lines demonstrated normal estrus cycles, fecundity, kisspeptin responsiveness, and inducible LH surge. We found no evidence of reproductive disruption in constant darkness. Overall, our results indicate that while conditional Bmal1 knockout in AVP, VIP, or NMS neurons is sufficient to disrupted locomotor activity, this disruption is insufficient to recapitulate the neuroendocrine reproductive effects of the whole-body Bmal1 knockout.
Keywords: arginine vasopressin; circadian clock; fertility; luteinizing hormone surge; neuromedin-s; suprachiasmatic nucleus; vasoactive intestinal peptide.
Copyright © 2022 Tonsfeldt, Cui, Lee, Walbeek, Brusman, Jin, Mieda, Gorman and Mellon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD100580/HD/NICHD NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- K99 NS119291/NS/NINDS NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- F32 HD090837/HD/NICHD NIH HHS/United States
- R01 HD072754/HD/NICHD NIH HHS/United States
- R01 HD082567/HD/NICHD NIH HHS/United States
- T32 HD007203/HD/NICHD NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- P50 HD012303/HD/NICHD NIH HHS/United States
- R24 HD102061/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

